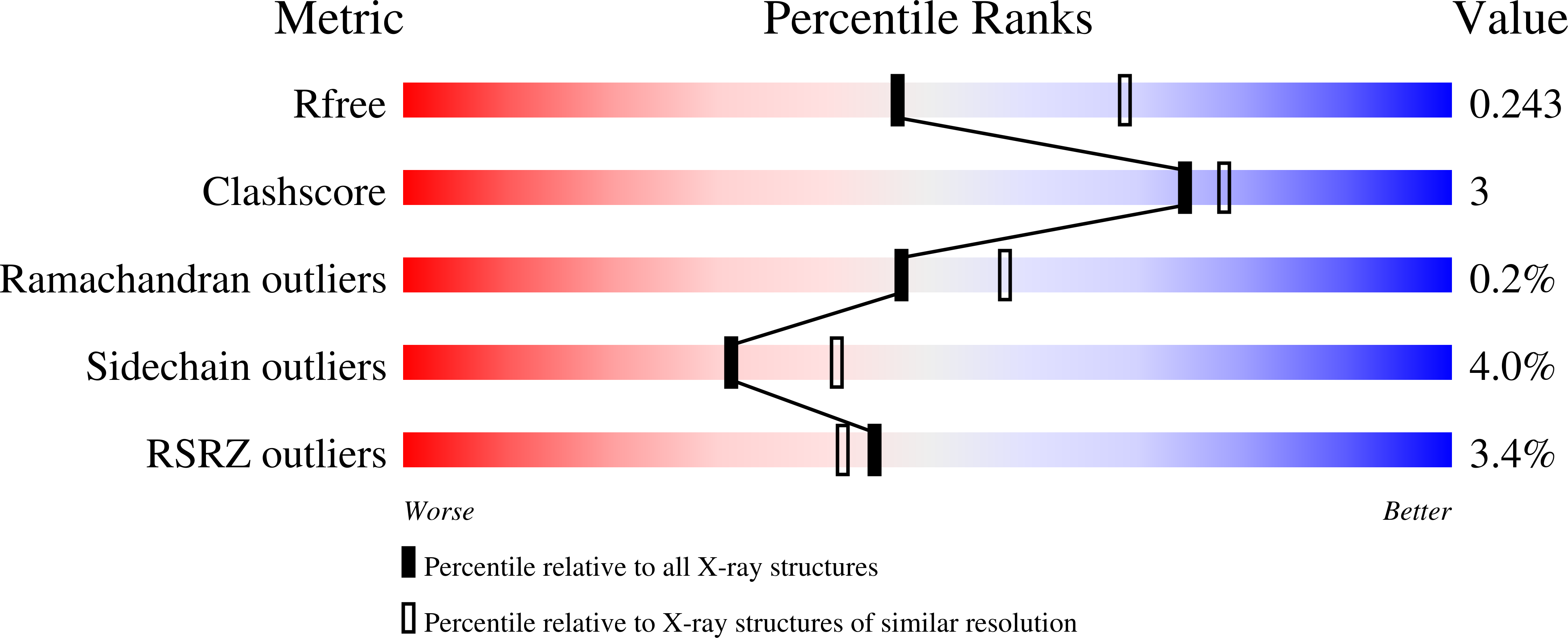
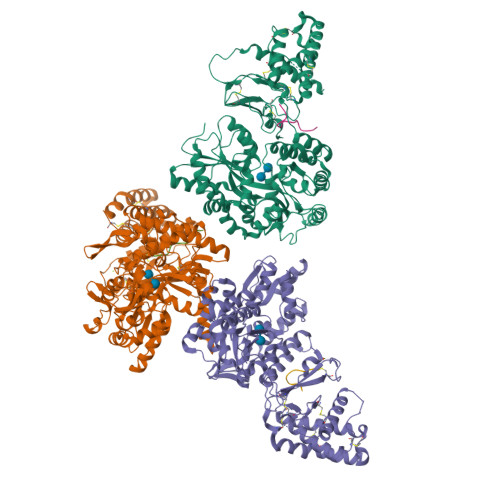
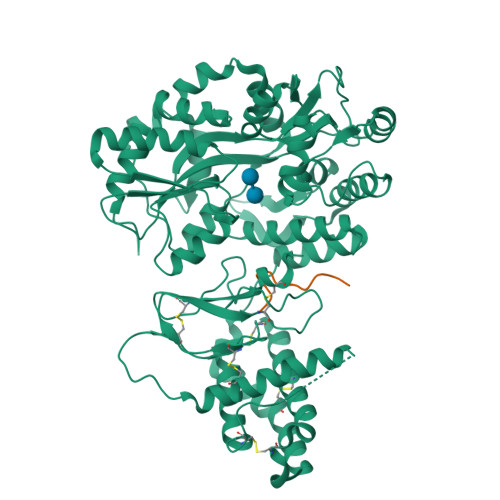
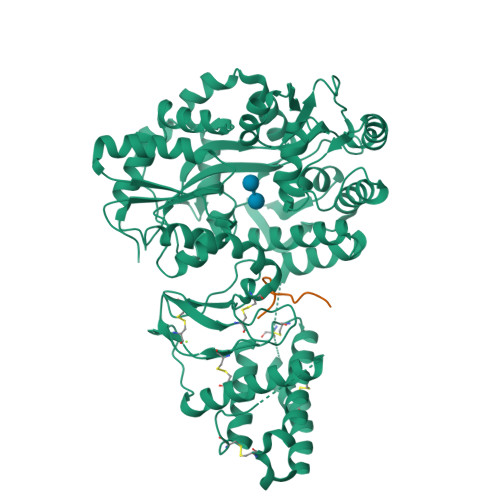
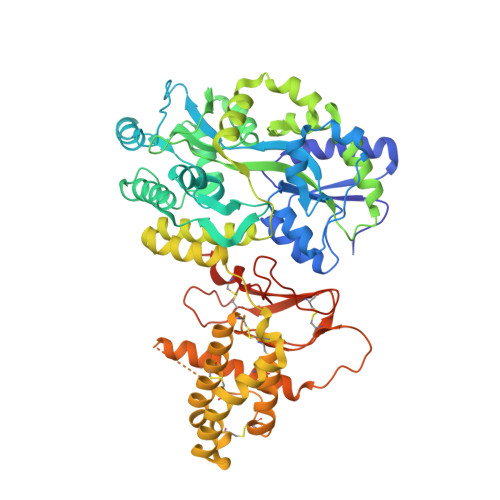
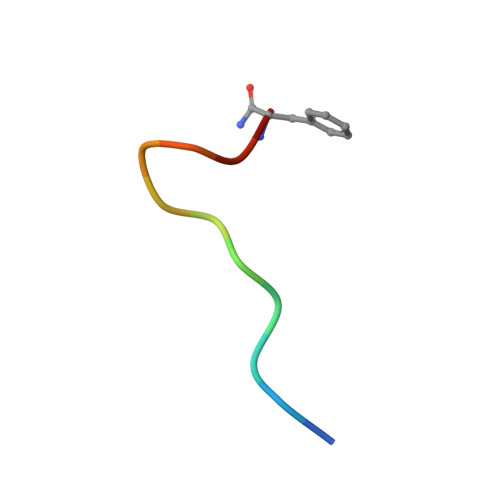
Structural Basis for Receptor Activity-Modifying Protein-Dependent Selective Peptide Recognition by a G Protein-Coupled Receptor.
Booe, J.M., Walker, C.S., Barwell, J., Kuteyi, G., Simms, J., Jamaluddin, M.A., Warner, M.L., Bill, R.M., Harris, P.W., Brimble, M.A., Poyner, D.R., Hay, D.L., Pioszak, A.A.(2015) Mol Cell 58: 1-13
- PubMed: 25982113
- DOI: https://doi.org/10.1016/j.molcel.2015.04.018
- Primary Citation of Related Structures:
4RWF, 4RWG - PubMed Abstract:
Association of receptor activity-modifying proteins (RAMP1-3) with the G protein-coupled receptor (GPCR) calcitonin receptor-like receptor (CLR) enables selective recognition of the peptides calcitonin gene-related peptide (CGRP) and adrenomedullin (AM) that have diverse functions in the cardiovascular and lymphatic systems. How peptides selectively bind GPCR:RAMP complexes is unknown. We report crystal structures of CGRP analog-bound CLR:RAMP1 and AM-bound CLR:RAMP2 extracellular domain heterodimers at 2.5 and 1.8 Å resolutions, respectively. The peptides similarly occupy a shared binding site on CLR with conformations characterized by a β-turn structure near their C termini rather than the α-helical structure common to peptides that bind related GPCRs. The RAMPs augment the binding site with distinct contacts to the variable C-terminal peptide residues and elicit subtly different CLR conformations. The structures and accompanying pharmacology data reveal how a class of accessory membrane proteins modulate ligand binding of a GPCR and may inform drug development targeting CLR:RAMP complexes.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.