An autoinhibited structure of alpha-catenin and its implications for vinculin recruitment to adherens junctions.
Ishiyama, N., Tanaka, N., Abe, K., Yang, Y.J., Abbas, Y.M., Umitsu, M., Nagar, B., Bueler, S.A., Rubinstein, J.L., Takeichi, M., Ikura, M.(2013) J Biological Chem 288: 15913-15925
- PubMed: 23589308
- DOI: https://doi.org/10.1074/jbc.M113.453928
- Primary Citation of Related Structures:
4K1N, 4K1O - PubMed Abstract:
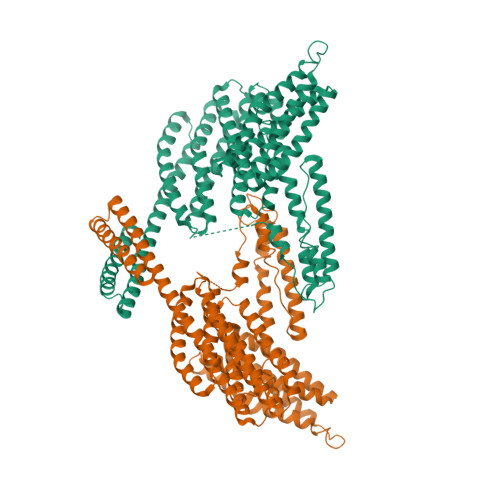
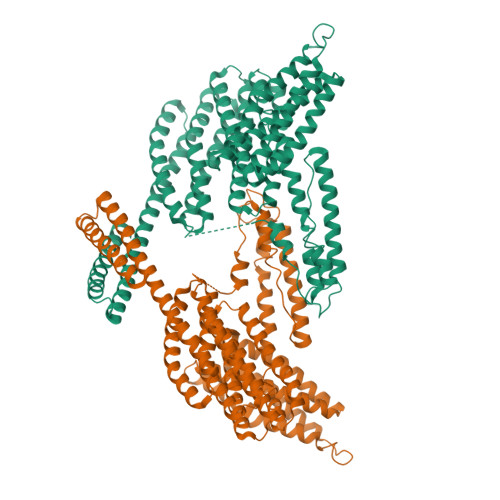
α-Catenin is an actin- and vinculin-binding protein that regulates cell-cell adhesion by interacting with cadherin adhesion receptors through β-catenin, but the mechanisms by which it anchors the cadherin-catenin complex to the actin cytoskeleton at adherens junctions remain unclear. Here we determined crystal structures of αE-catenin in the autoinhibited state and the actin-binding domain of αN-catenin. Together with the small-angle x-ray scattering analysis of full-length αN-catenin, we deduced an elongated multidomain assembly of monomeric α-catenin that structurally and functionally couples the vinculin- and actin-binding mechanisms. Cellular and biochemical studies of αE- and αN-catenins show that αE-catenin recruits vinculin to adherens junctions more effectively than αN-catenin, partly because of its higher affinity for actin filaments. We propose a molecular switch mechanism involving multistate conformational changes of α-catenin. This would be driven by actomyosin-generated tension to dynamically regulate the vinculin-assisted linkage between adherens junctions and the actin cytoskeleton.
Organizational Affiliation:
Campbell Family Cancer Research Institute, Ontario Cancer Institute, Toronto, Ontario M5G 1L7, Canada.