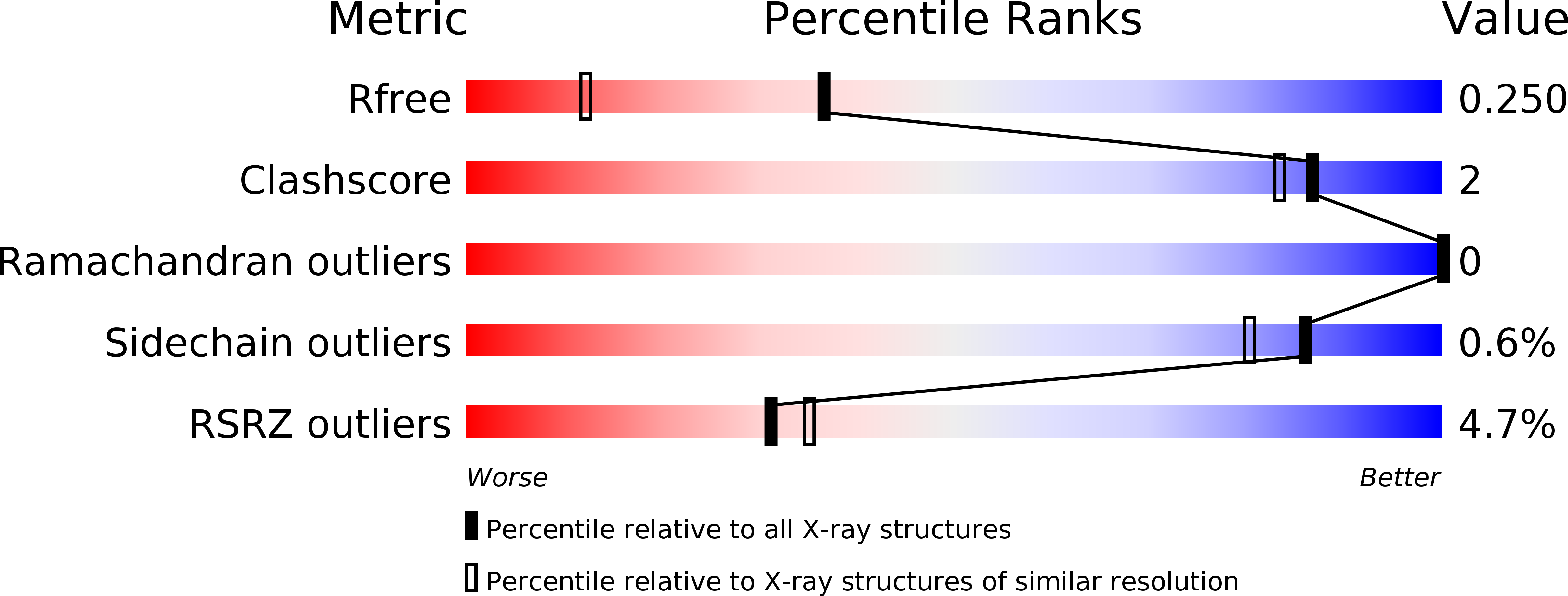
Structural and functional properties of staphylococcal superantigen-like protein 4
Hermans, S.J., Baker, H.M., Sequeira, R.P., Langley, R.J., Baker, E.N., Fraser, J.D.(2012) Infect Immun 80: 4004-4013
- PubMed: 22949551
- DOI: https://doi.org/10.1128/IAI.00764-12
- Primary Citation of Related Structures:
4DXF, 4DXG - PubMed Abstract:
Staphylococcus aureus is a prevalent and significant human pathogen. Among the repertoire of virulence factors produced by this bacterium are the 14 staphylococcal superantigen-like (SSL) proteins. SSL protein 4 (SSL4) is one member of this family and contains a highly conserved carbohydrate binding site also found in SSL2, SSL3, SSL5, SSL6, and SSL11. Recombinant SSL4(t), comprising amino acids 109 to 309 of Newman strain SSL4 (SSL4-Newman), has been shown to bind and be internalized by human granulocytes and macrophages in a sialic-acid (Sia)-dependent manner. SSL4(t) can compete with itself for cell binding, indicating that binding is target specific. A 2.5-Å-resolution crystal structure of SSL4(t) complexed with sialyl Lewis X (sLe(x)) [sLe(x)-Neu5Acα2-3Galβ1-4(Fucα1-3)GlcNAc] revealed a similar binding site to SSL5 and SSL11. These data, along with data on SSL4(t) binding to a glycan array and biosensor analysis of sLe(x) and sialyllactosamine (sLacNac) binding are compared with those for SSL11. Although these proteins show great similarity in their carbohydrate binding sites, with a root mean square (RMS) difference between main chain atom positions of only 0.34 Å, these proteins differ in detail in their affinity for sLe(x) and sLacNac, as well as their glycan preference. Together with cell binding data, this shows how S. aureus produces multiple related proteins that target myeloid cells through specific sialyllactosamine-containing glycoproteins.
Organizational Affiliation:
School of Medical Sciences, University of Auckland, Aukland, New Zealand.