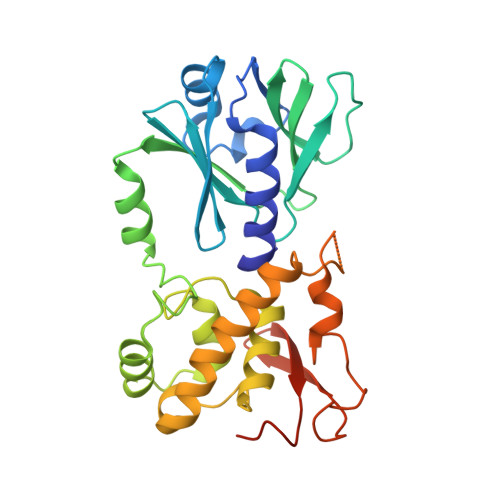
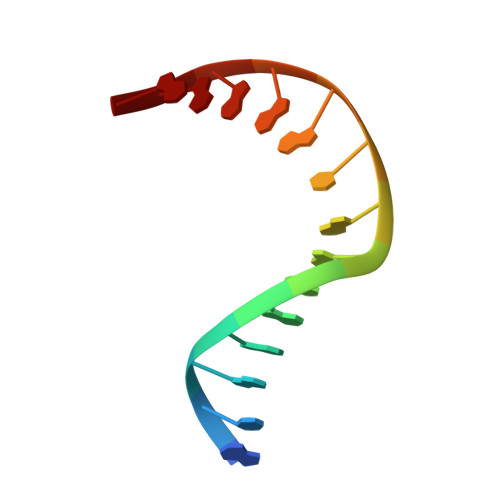
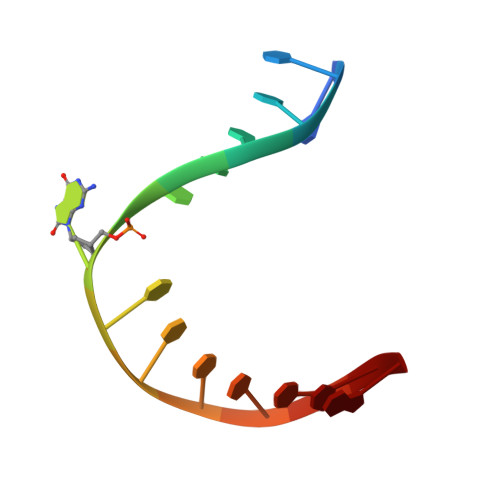
Ribose-protonated DNA base excision repair: a combined theoretical and experimental study.
Sadeghian, K., Flaig, D., Blank, I.D., Schneider, S., Strasser, R., Stathis, D., Winnacker, M., Carell, T., Ochsenfeld, C.(2014) Angew Chem Int Ed Engl 53: 10044-10048
- PubMed: 25065673
- DOI: https://doi.org/10.1002/anie.201403334
- Primary Citation of Related Structures:
4CIS - PubMed Abstract:
Living organisms protect the genome against external influences by recognizing and repairing damaged DNA. A common source of gene mutation is the oxidized guanine, which undergoes base excision repair through cleavage of the glycosidic bond between the ribose and the nucleobase of the lesion. We unravel the repair mechanism utilized by bacterial glycosylase, MutM, using quantum-chemical calculations involving more than 1000 atoms of the catalytic site. In contrast to the base-protonated pathway currently favored in the literature, we show that the initial protonation of the lesion's ribose paves the way for an almost barrier-free glycosidic cleavage. The combination of theoretical and experimental data provides further insight into the selectivity and discrimination of MutM's binding site toward various substrates.
Organizational Affiliation:
Chair of Theoretical Chemistry, Department of Chemistry, University of Munich (LMU), Butenandtstrasse 7, 81377 Munich (Germany); Center for Integrated Protein Science (CIPSM) at the Department of Chemistry, University of Munich (LMU), Butenandtstrasse 5-13, 81377 Munich (Germany).