SMY2-type GYF domain recognition in mRNA surveillance complexes
Ash, M.R., Faelber, K., Kosslick, D., Albert, G., Roske, Y., Kofler, M., Schuemann, M., Krause, E., Freund, C.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein SMY2 | 100 | Saccharomyces cerevisiae | Mutation(s): 0 Gene Names: SMY2 | 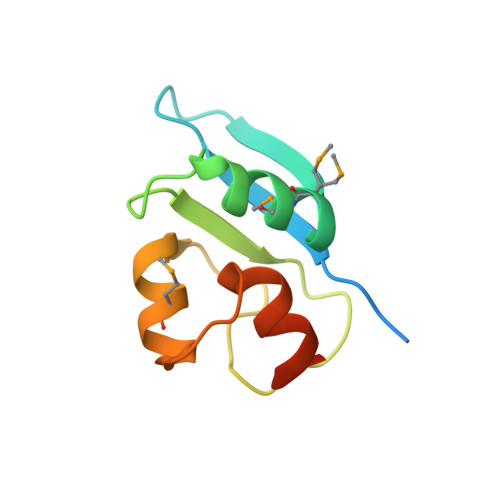 | |
UniProt | |||||
Find proteins for P32909 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P32909 Go to UniProtKB: P32909 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P32909 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Branchpoint-bridging protein | F [auth L], G [auth M], H [auth N], I [auth O], J [auth P] | 11 | N/A | Mutation(s): 0 | 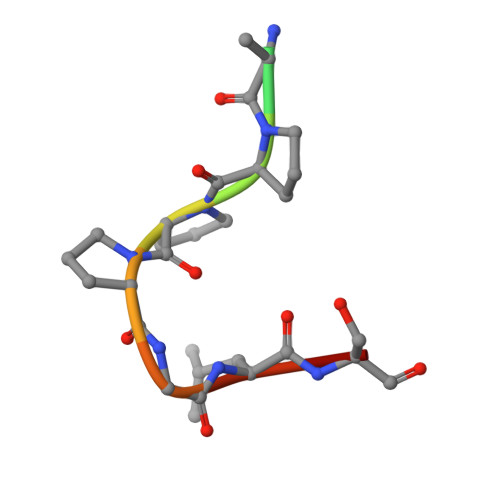 |
UniProt | |||||
Find proteins for Q12186 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore Q12186 Go to UniProtKB: Q12186 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q12186 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B, C, D, E | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 101.4 | α = 90 |
| b = 101.4 | β = 90 |
| c = 150.7 | γ = 90 |
| Software Name | Purpose |
|---|---|
| MOSFLM | data reduction |
| SCALA | data scaling |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| SHELXCD | phasing |
| SHELXE | model building |