Unconserved substrate-binding sites direct the stereoselectivity of medium-chain alcohol dehydrogenase
Wang, S.S., Nie, Y., Xu, Y., Zhang, R.Z., Ko, T.P., Huang, C.H., Chan, H.C., Guo, R.T., Xiao, R.(2014) Chem Commun (Camb) 50: 7770-7772
- PubMed: 24834985
- DOI: https://doi.org/10.1039/c4cc01752h
- Primary Citation of Related Structures:
3WLE, 3WLF, 3WNQ - PubMed Abstract:
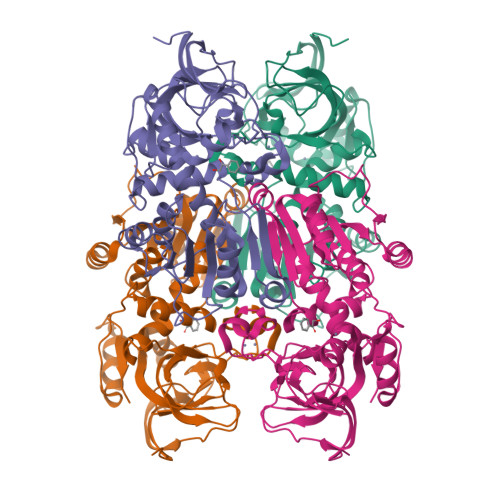
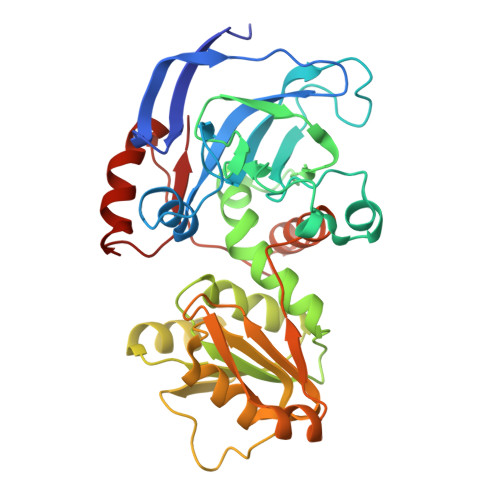
Structure-guided design of substrate-binding pocket inversed the stereoselectivity of an NADH-dependent medium-chain alcohol dehydrogenase (MDR) from Prelog to anti-Prelog. The pocket-forming amino acids, especially the unconserved residues as hotspots, play critical roles in directing MDRs' stereoselectivity.
Organizational Affiliation:
School of Biotechnology and Key Laboratory of Industrial Biotechnology, Ministry of Education, Jiangnan University, Wuxi 214122, China. yxu@jiangnan.edu.cn.