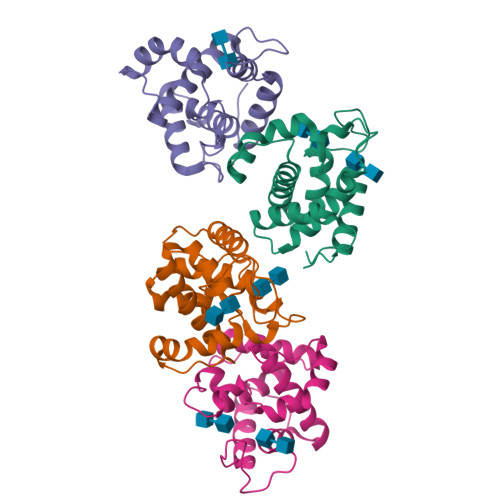
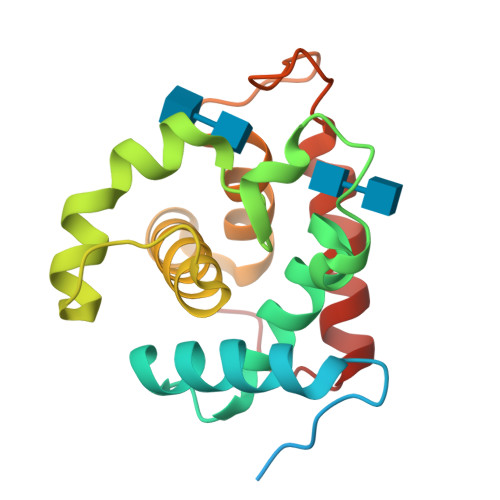
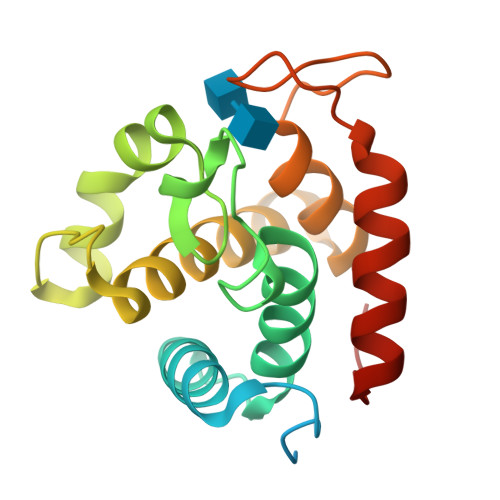
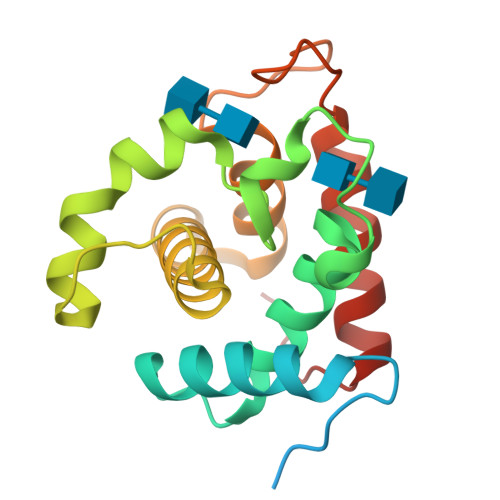
Crystal Structures of the Catalytic Domain of a Novel Glycohydrolase Family 23 Chitinase from Ralstonia sp. A-471 Reveals a Unique Arrangement of the Catalytic Residues for Inverting Chitin Hydrolysis
Arimori, T., Kawamoto, N., Shinya, S., Okazaki, N., Nakazawa, M., Miyatake, K., Fukamizo, T., Ueda, M., Tamada, T.(2013) J Biological Chem 288: 18696-18706
- PubMed: 23658014
- DOI: https://doi.org/10.1074/jbc.M113.462135
- Primary Citation of Related Structures:
3W6B, 3W6C, 3W6D, 3W6E, 3W6F - PubMed Abstract:
Chitinase C from Ralstonia sp. A-471 (Ra-ChiC) has a catalytic domain sequence similar to goose-type (G-type) lysozymes and, unlike other chitinases, belongs to glycohydrolase (GH) family 23. Using NMR spectroscopy, however, Ra-ChiC was found to interact only with the chitin dimer but not with the peptidoglycan fragment. Here we report the crystal structures of wild-type, E141Q, and E162Q of the catalytic domain of Ra-ChiC with or without chitin oligosaccharides. Ra-ChiC has a substrate-binding site including a tunnel-shaped cavity, which determines the substrate specificity. Mutation analyses based on this structural information indicated that a highly conserved Glu-141 acts as a catalytic acid, and that Asp-226 located at the roof of the tunnel activates a water molecule as a catalytic base. The unique arrangement of the catalytic residues makes a clear contrast to the other GH23 members and also to inverting GH19 chitinases.
Organizational Affiliation:
Quantum Beam Science Directorate, Japan Atomic Energy Agency, 2-4 Shirakata-Shirane, Tokai, Ibaraki 319-1195, Japan.