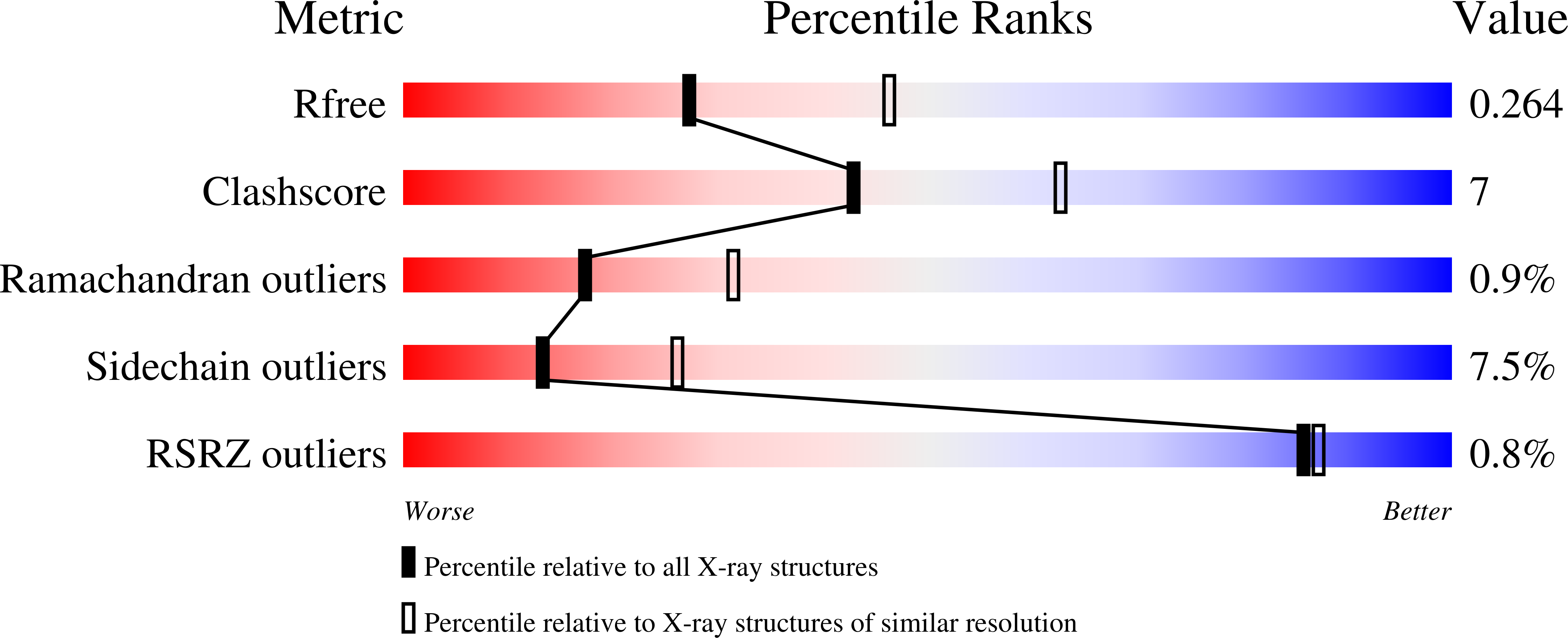
Structures of aminophenol dioxygenase in complex with intermediate, product and inhibitor
Li, D.F., Zhang, J.Y., Hou, Y.J., Liu, L., Hu, Y., Liu, S.J., Wang, D.C., Liu, W.(2013) Acta Crystallogr D Biol Crystallogr 69: 32-43
- PubMed: 23275161
- DOI: https://doi.org/10.1107/S0907444912042072
- Primary Citation of Related Structures:
3VSG, 3VSH, 3VSI, 3VSJ - PubMed Abstract:
Dioxygen activation by nonhaem Fe(II) enzymes containing the 2-His-1-carboxylate facial triad has been extensively studied in recent years. Here, crystal structures of 2-aminophenol 1,6-dioxygenase, an enzyme that represents a minor group of extradiol dioxygenases and that catalyses the ring opening of 2-aminophenol, in complex with the lactone intermediate (4Z,6Z)-3-iminooxepin-2(3H)-one and the product 2-aminomuconic 6-semialdehyde and in complex with the suicide inhibitor 4-nitrocatechol are reported. The Fe-ligand binding schemes observed in these structures revealed some common geometrical characteristics that are shared by the published structures of extradiol dioxygenases, suggesting that enzymes that catalyse the oxidation of noncatecholic compounds are very likely to utilize a similar strategy for dioxygen activation and the fission of aromatic rings as the canonical mechanism. The Fe-ligation arrangement, however, is strikingly enantiomeric to that of all other 2-His-1-carboxylate enzymes apart from protocatechuate 4,5-dioxygenase. This structural variance leads to the generation of an uncommon O(-)-Fe(2+)-O(-) species prior to O(2) binding, which probably forms the structural basis on which APD distinguishes its specific substrate and inhibitor, which share an analogous molecular structure.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, People's Republic of China.