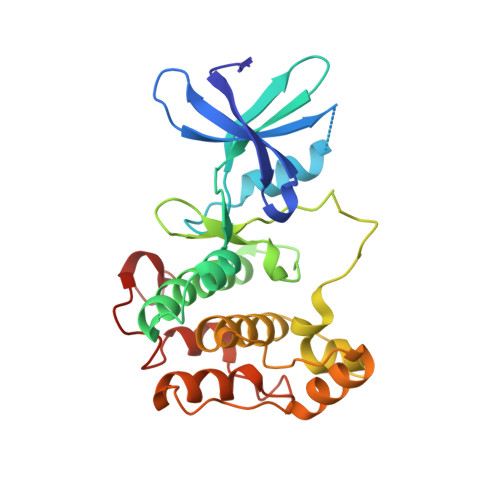
An inhibited conformation for the protein kinase domain of the Saccharomyces cerevisiae AMPK homolog Snf1.
Rudolph, M.J., Amodeo, G.A., Tong, L.(2010) Acta Crystallogr Sect F Struct Biol Cryst Commun 66: 999-1002
- PubMed: 20823513
- DOI: https://doi.org/10.1107/S1744309110028265
- Primary Citation of Related Structures:
3MN3 - PubMed Abstract:
AMP-activated protein kinase (AMPK) is a master metabolic regulator for controlling cellular energy homeostasis. Its homolog in yeast, SNF1, is activated in response to glucose depletion and other stresses. The catalytic (alpha) subunit of AMPK/SNF1 in yeast (Snf1) contains a protein Ser/Thr kinase domain (KD), an auto-inhibitory domain (AID) and a region that mediates interactions with the two regulatory (beta and gamma) subunits. Here, the crystal structure of residues 41-440 of Snf1, which include the KD and AID, is reported at 2.4 A resolution. The AID is completely disordered in the crystal. A new inhibited conformation of the KD is observed in a DFG-out conformation and with the glycine-rich loop adopting a structure that blocks ATP binding to the active site.
Organizational Affiliation:
Department of Biological Sciences, Columbia University, New York, NY 10027, USA.