Crystal structure of the co-expressed succinyl-CoA transferase A and B complex from Bacillus subtilis.
Kim, Y., Zhou, M., Stols, L., Eschenfeldt, W., Donnelly, M., Joachimiak, A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Succinyl-CoA:3-ketoacid-coenzyme A transferase subunit A | 241 | Bacillus subtilis | Mutation(s): 0 Gene Names: scoA, yxjD, BSU38990, N15K EC: 2.8.3.5 | 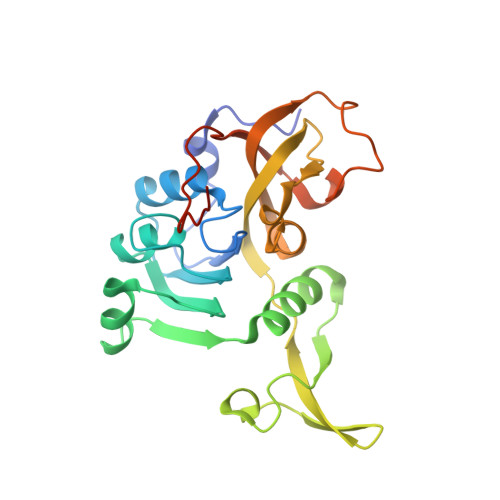 | |
UniProt | |||||
Find proteins for P42315 (Bacillus subtilis (strain 168)) Explore P42315 Go to UniProtKB: P42315 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P42315 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Succinyl-CoA:3-ketoacid-coenzyme A transferase subunit B | 219 | Bacillus subtilis | Mutation(s): 0 Gene Names: scoB, yxjE, BSU38980, N15L EC: 2.8.3.5 | 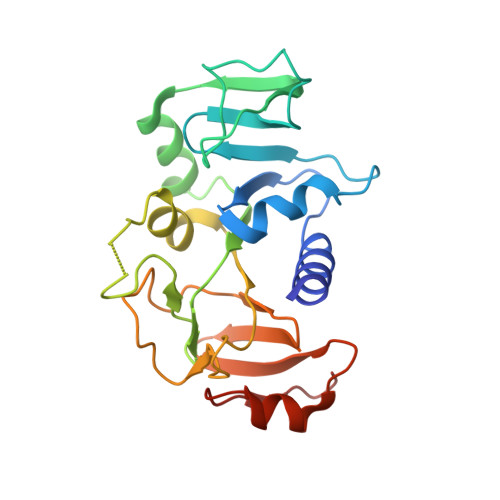 | |
UniProt | |||||
Find proteins for P42316 (Bacillus subtilis (strain 168)) Explore P42316 Go to UniProtKB: P42316 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P42316 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 69.337 | α = 90 |
| b = 70.404 | β = 106.31 |
| c = 97.996 | γ = 90 |
| Software Name | Purpose |
|---|---|
| MOLREP | phasing |
| Coot | model building |
| REFMAC | refinement |
| SBC-Collect | data collection |
| HKL-2000 | data collection |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |