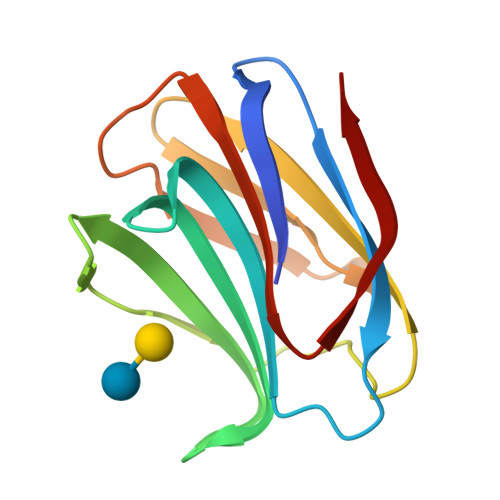
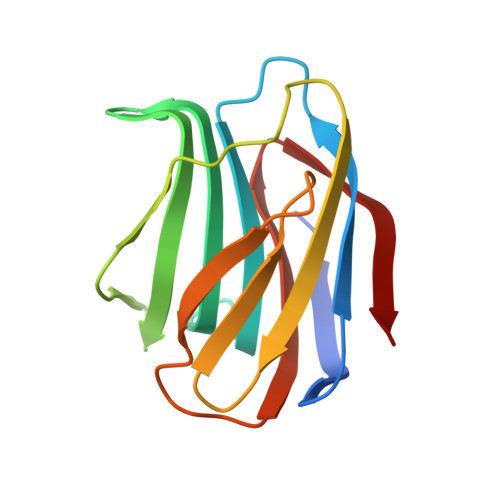
Tracing protein evolution through ancestral structures of fish galectin
Konno, A., Kitagawa, A., Watanabe, M., Ogawa, T., Shirai, T.(2011) Structure 19: 711-721
- PubMed: 21565705
- DOI: https://doi.org/10.1016/j.str.2011.02.014
- Primary Citation of Related Structures:
3AJY, 3AJZ, 3AK0 - PubMed Abstract:
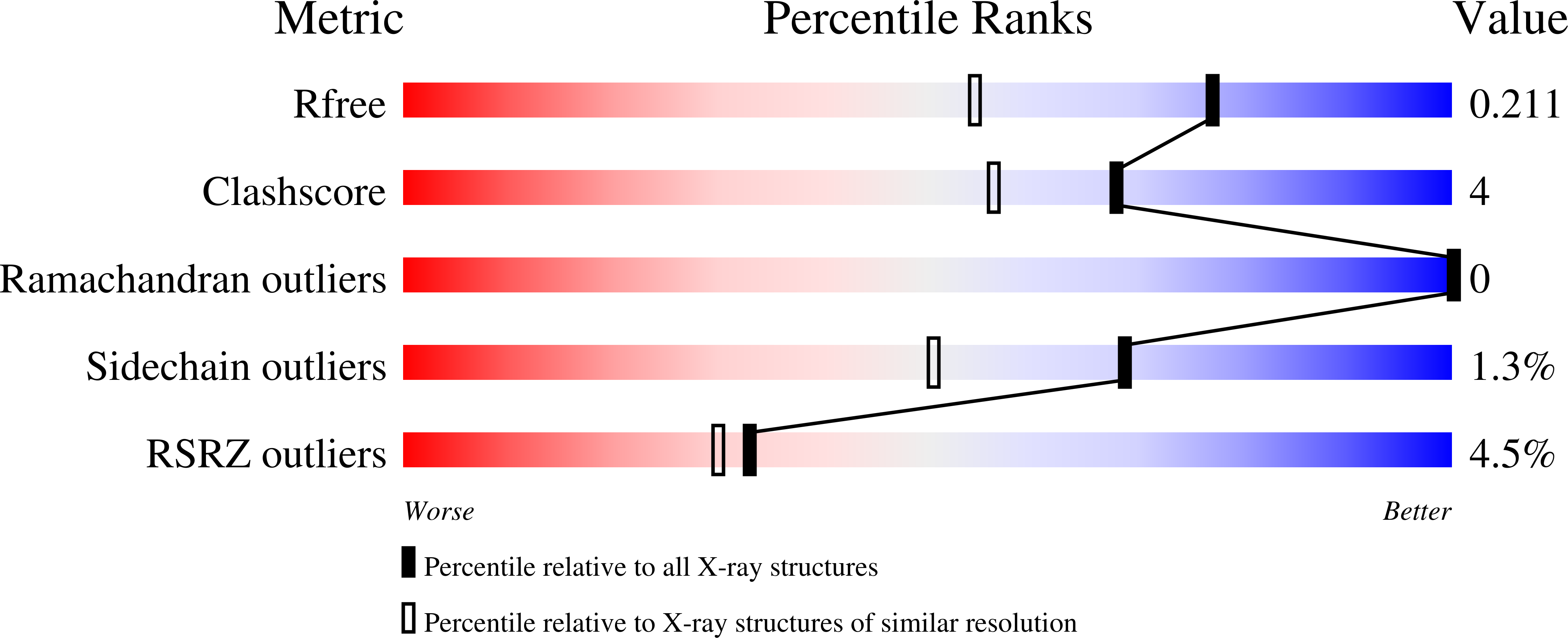
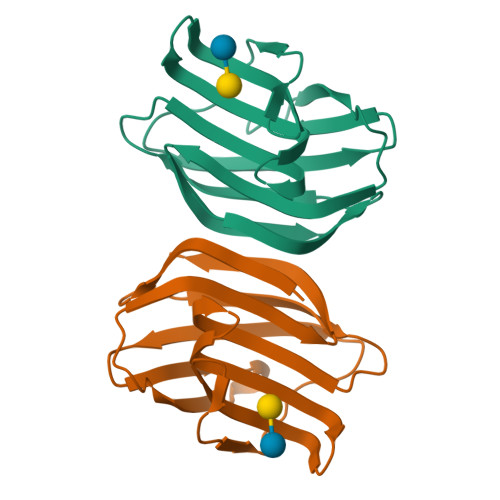
Ancestral structures of fish galectins (congerins) were determined. The extant isoforms I and II of congerin are the components of a fish biological defense system and have rapidly differentiated under natural selection pressure, by which congerin I has experienced a protein-fold evolution. The dimer structure of the ancestral congerin demonstrated intermediate features of the extant isoforms. The protein-fold evolution was not observed in the ancestral structure, indicating it specifically occurred in congerin I lineage. Details of hydrogen bonding pattern at the dimer interface and the carbohydrate-binding site of the ancestor were different from the current proteins. The differences implied these proteins were under selection pressure for stabilizing dimer structure and differentiation in carbohydrate specificity. The ancestor had rather low cytotoxic activity than offspring, indicating selection was made to enhance this activity of congerins. Combined with functional analyses, the structure revealed atomic details of the differentiation process of the proteins.
Organizational Affiliation:
Department of Biomolecular Science, Graduate School of Life Sciences, Tohoku University, Sendai 980-8577, Japan.