Chemical Synthesis, Folding, and Structural Insights into O-Fucosylated Epidermal Growth Factor-like Repeat 12 of Mouse Notch-1 Receptor
Hiruma-Shimizu, K., Hosoguchi, K., Liu, Y., Fujitani, N., Ohta, T., Hinou, H., Matsushita, T., Shimizu, H., Feizi, T., Nishimura, S.(2010) J Am Chem Soc 132: 14857-14865
- PubMed: 20883017
- DOI: https://doi.org/10.1021/ja105216u
- Primary Citation of Related Structures:
2RQZ, 2RR0, 2RR2 - PubMed Abstract:
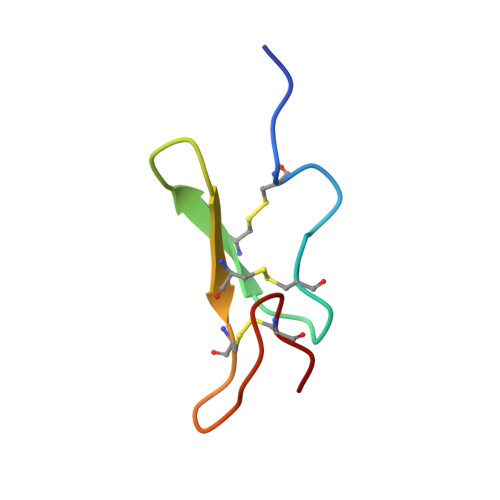
Notch receptors are cell surface glycoproteins that play key roles in a number of developmental cascades in metazoa. The extracellular domains of Notch-1 receptors are composed of 36 tandem epidermal growth factor (EGF)-like repeats, many of which are modified at highly conserved consensus sites by an unusual form of O-glycan, with O-fucose. The O-fucose residues on certain EGF repeats may be elongated. In mammalian cells this can be a tetrasaccharide, Siaα2,3Galβ1,4GlcNAcβ1,3Fucα1→. This elongation process is initiated by the action of O-fucose-specific β1,3 N-acetylglucosaminyltransferases of the Fringe family. There is evidence that the addition of GlcNAc by Fringe serves as an essential modulator of the interaction of Notch with its ligands and the triggering of activation. Here we describe the efficient synthesis, folding, and structural characterization of EGF repeat 12 (EGF 12) of a mouse Notch-1 receptor bearing different O-fucose glycan chains. We demonstrate that the three disulfide bonds, Cys(456)-Cys(467) (C1-C3), Cys(461)-Cys(476) (C2-C4), and Cys(478)-Cys(487) (C5-C6) were correctly formed in the nonglycosylated as well as the O-fucosylated forms of EGF 12. Three-dimensional structural studies by NMR reveal that the methyl group of fucose is in close contact with ILe(475), Met(477), Pro(478) residues and this stabilizes the conformation of the antiparallel β-sheet of EGF 12. The addition of the GlcNAc residue on O-fucosylated EGF 12 induces a significant conformational change in the adjacent tripeptide sequence, Gln(462)Asn(463)Asp(464), which is a motif involved in the natural, enzymatic O-fucosylation at the conserved site (Cys(461)X(4)Ser/ThrCys(467)).
Organizational Affiliation:
Graduate School of Life Science, Frontier Research Center for Post-Genomic Science and Technology, Hokkaido University, N21, W11, Sapporo 001-0021, Japan, National Institute of Advanced Industrial Science and Technology (AIST), Sapporo 062-8517, Japan, and Glycosciences Laboratory, Imperial College London, Northwick Park Campus, Harrow, Middlesex, HA1 3UJ, U.K.