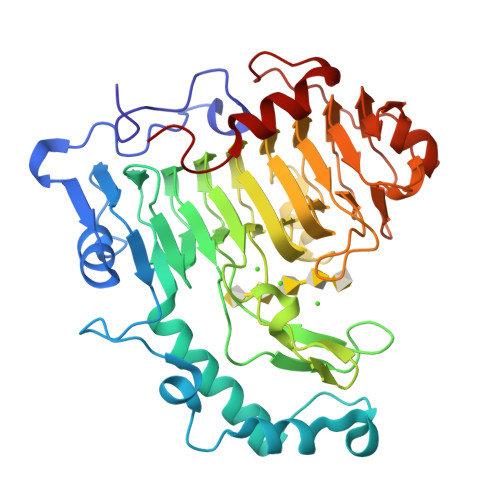
Structural insights into substrate specificity and the anti beta-elimination mechanism of pectate lyase.
Seyedarabi, A., To, T.T., Ali, S., Hussain, S., Fries, M., Madsen, R., Clausen, M.H., Teixteira, S., Brocklehurst, K., Pickersgill, R.W.(2010) Biochemistry 49: 539-546
- PubMed: 20000851
- DOI: https://doi.org/10.1021/bi901503g
- Primary Citation of Related Structures:
2NZM, 2O04, 2O0V, 2O17, 2O1D, 3KRG - PubMed Abstract:
Pectate lyases harness anti beta-elimination chemistry to cleave the alpha-1,4 linkage in the homogalacturonan region of plant cell wall pectin. We have studied the binding of five pectic oligosaccharides to Bacillus subtilis pectate lyase in crystals of the inactive enzyme in which the catalytic base is substituted with alanine (R279A). We discover that the three central subsites (-1, +1, and +2) have a profound preference for galacturonate but that the distal subsites can accommodate methylated galacturonate. It is reasonable to assume therefore that pectate lyase can cleave pectin with three consecutive galacturonate residues. The enzyme in the absence of substrate binds a single calcium ion, and we show that two additional calcium ions bind between enzyme and substrate carboxylates occupying the +1 subsite in the Michaelis complex. The substrate binds less intimately to the enzyme in a complex made with a catalytic base in place but in the absence of the calcium ions and an adjacent lysine. In this complex, the catalytic base is correctly positioned to abstract the C5 proton, but there are no calcium ions binding the carboxylate at the +1 subsite. It is clear, therefore, that the catalytic calcium ions and adjacent lysine promote catalysis by acidifying the alpha-proton, facilitating its abstraction by the base. There is also clear evidence that binding distorts the relaxed 2(1) or 3(1) helical conformation of the oligosaccharides in the region of the scissile bond.
Organizational Affiliation:
School of Biological and Chemical Sciences, Queen Mary University of London, Mile End Road, London E1 4NS, United Kingdom.