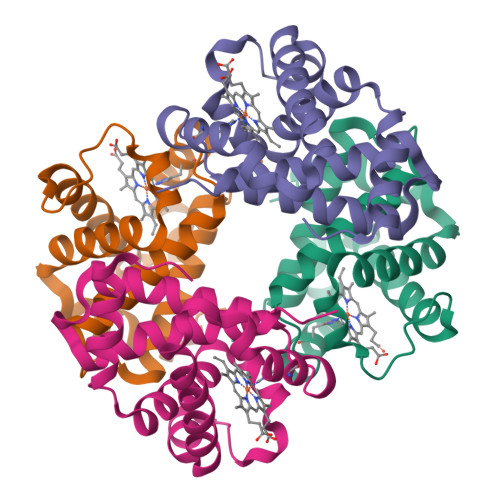
Protonation states of buried histidine residues in human deoxyhemoglobin revealed by neutron crystallography.
Chatake, T., Shibayama, N., Park, S.Y., Kurihara, K., Tamada, T., Tanaka, I., Niimura, N., Kuroki, R., Morimoto, Y.(2007) J Am Chem Soc 129: 14840-14841
- PubMed: 17990881
- DOI: https://doi.org/10.1021/ja0749441
- Primary Citation of Related Structures:
2DXM - PubMed Abstract:
The protonation states of buried histidine residues in human deoxyhemoglobin were unambiguously identified by using a neutron crystallographic technique. Unexpectedly, the neutron structure reveals that both the alpha- and beta-distal histidines (Hisalpha58 and Hisbeta63) adopt a positively charged, fully (doubly) protonated form, suggesting their contribution to the Bohr effect. In addition, the neutron data provide an accurate picture of the alpha1beta1 hydrogen-bonding network and allow us to observe unambiguously the nature of the intradimeric interactions at an atomic level.
Organizational Affiliation:
Research Reactor Institute, Kyoto University, Kumatori, Osaka 590-0494, Japan.