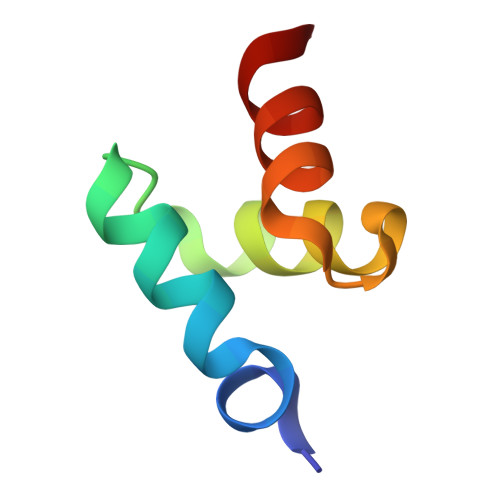
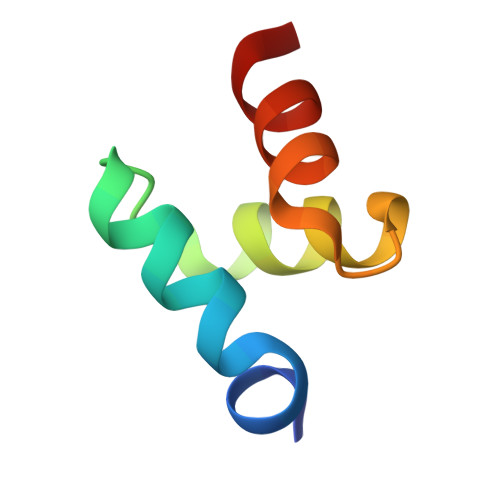
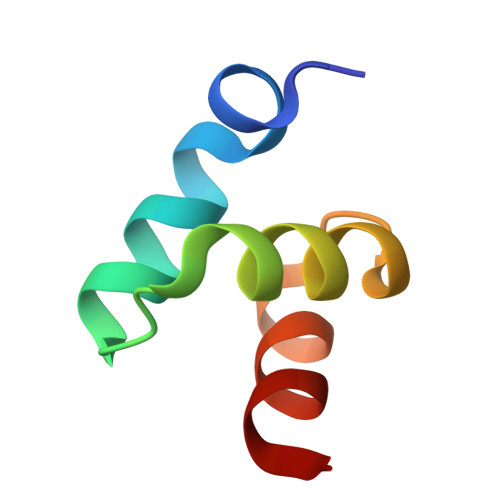
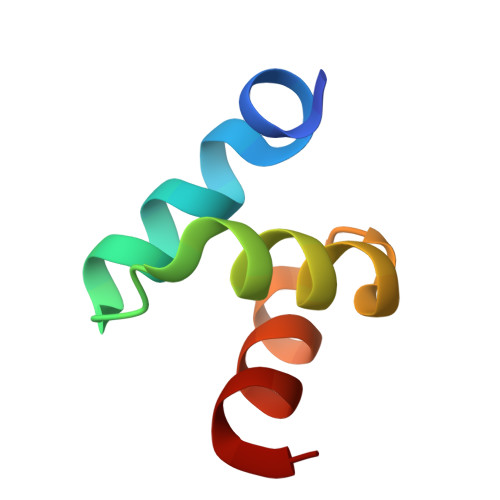
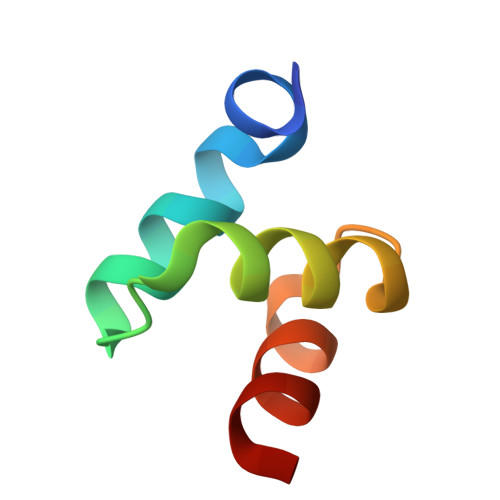
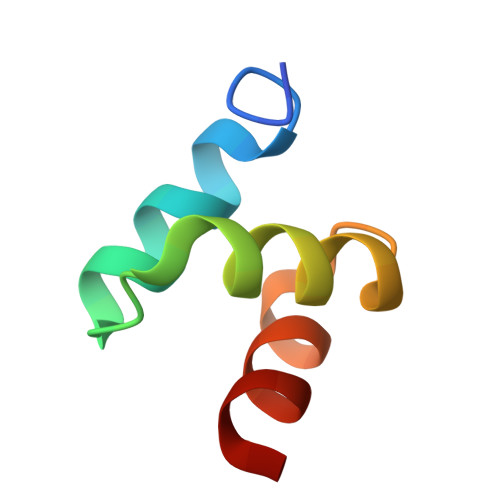
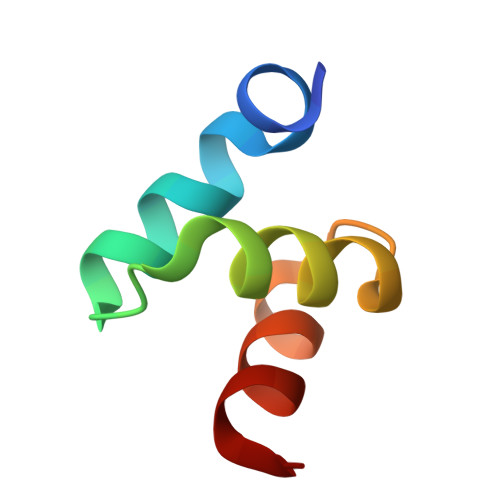
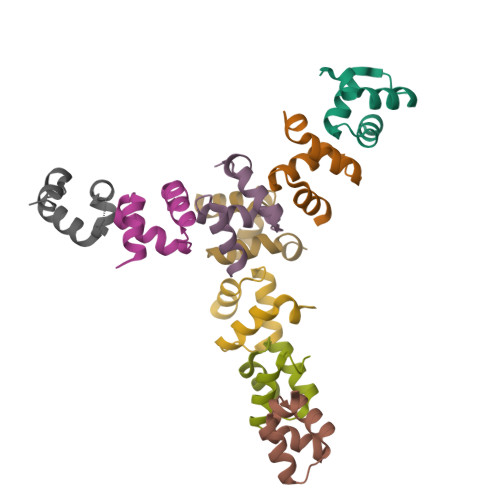Structures of the Dsk2 Ubl and Uba Domains and Their Complex.
Lowe, E.D., Hasan, N., Trempe, J.-F., Fonso, L., Noble, M.E.M., Endicott, J.A., Johnson, L.N., Brown, N.R.(2006) Acta Crystallogr D Biol Crystallogr 62: 177
- PubMed: 16421449
- DOI: https://doi.org/10.1107/S0907444905037777
- Primary Citation of Related Structures:
2BWB, 2BWE, 2BWF - PubMed Abstract:
The yeast proteins Dsk2 and Rad23 belong to a family of proteins that contain an N-terminal ubiquitin-like domain (UBL) and a C-terminal ubiquitin-associated domain (UBA). Both Dsk2 and Rad23 function as adaptors to target ubiquitin-labelled proteins to the proteasome through recognition of polyubiquitin (four or more K48-linked ubiquitins) by their UBA domains and to the yeast proteasomal subunit Rpn1 by their UBL domains. The crystal structures of the Dsk2 UBL domain, the Dsk2 UBA domain and the Dsk2 UBA-UBL complex are reported. In the crystal, the Dsk2 UBA domains associate through electrostatic interactions to form ninefold helical ribbons that leave the ubiquitin-binding surface exposed. The UBA-UBL complex explains the reduced affinity of the UBA domain for UBL compared with ubiquitin and has implications for the regulation of Dsk2 adaptor function during ubiquitin-mediated proteasomal targeting. A model is discussed in which two or more Dsk2 UBA molecules may selectively bind to K48-linked polyubiquitin.
Organizational Affiliation:
Laboratory of Molecular Biophysics, Department of Biochemistry, University of Oxford, Oxford OX1 3QU, England.