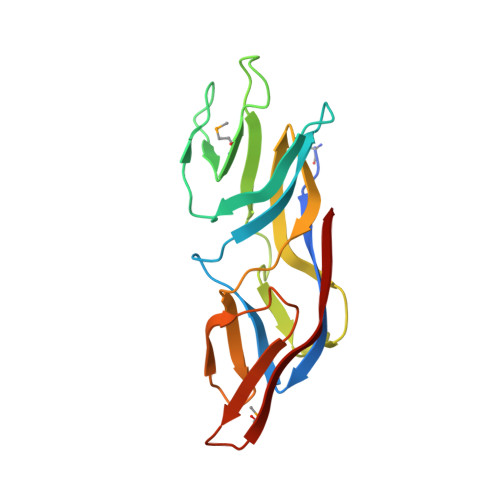Structure of the conserved hypothetical protein MAL13P1.257 from Plasmodium falciparum.
Holmes, M.A., Buckner, F.S., Van Voorhis, W.C., Mehlin, C., Boni, E., Earnest, T.N., DeTitta, G., Luft, J., Lauricella, A., Anderson, L., Kalyuzhniy, O., Zucker, F., Schoenfeld, L.W., Hol, W.G., Merritt, E.A.(2006) Acta Crystallogr Sect F Struct Biol Cryst Commun 62: 180-185
- PubMed: 16511296
- DOI: https://doi.org/10.1107/S1744309106005847
- Primary Citation of Related Structures:
1ZSO - PubMed Abstract:
The structure of a conserved hypothetical protein, PlasmoDB sequence MAL13P1.257 from Plasmodium falciparum, Pfam sequence family PF05907, has been determined as part of the structural genomics effort of the Structural Genomics of Pathogenic Protozoa consortium. The structure was determined by multiple-wavelength anomalous dispersion at 2.17 A resolution. The structure is almost entirely beta-sheet; it consists of 15 beta-strands and one short 3(10)-helix and represents a new protein fold. The packing of the two monomers in the asymmetric unit indicates that the biological unit may be a dimer.
Organizational Affiliation:
Structural Genomics of Pathogenic Protozoa Consortium, USA.