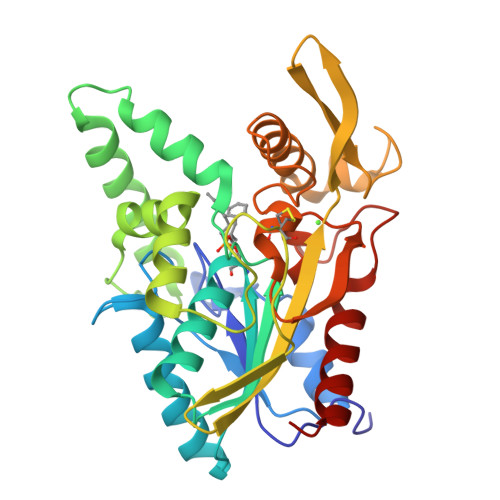
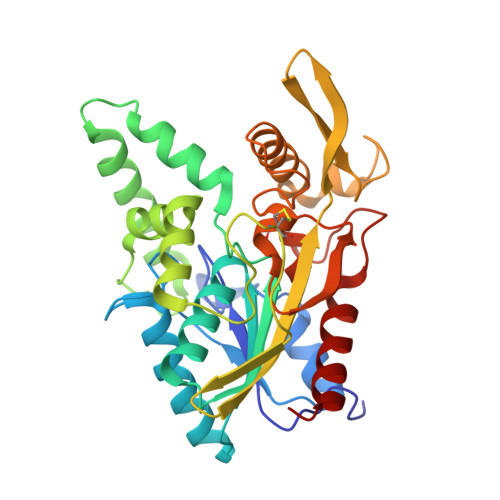
Mirror-Image Packing in Enantiomer Discrimination Molecular Basis for the Enantioselectivity of B.cepacia Lipase toward 2-Methyl-3-Phenyl-1-Propanol.
Mezzetti, A., Schrag, J.D., Cheong, C.S., Kazlauskas, R.J.(2005) Chem Biol 12: 427-437
- PubMed: 15850979
- DOI: https://doi.org/10.1016/j.chembiol.2005.01.016
- Primary Citation of Related Structures:
1YS1, 1YS2 - PubMed Abstract:
Synthetic chemists often exploit the high enantioselectivity of lipases to prepare pure enantiomers of primary alcohols, but the molecular basis for this enantioselectivity is unknown. The crystal structures of two phosphonate transition-state analogs bound to Burkholderia cepacia lipase reveal this molecular basis for a typical primary alcohol: 2-methyl-3-phenyl-1-propanol. The enantiomeric alcohol moieties adopt surprisingly similar orientations, with only subtle differences that make it difficult to predict how to alter enantioselectivity. These structures, along with a survey of previous structures of enzyme bound enantiomers, reveal that binding of enantiomers does not involve an exchange of two substituent positions as most researchers assumed. Instead, the enantiomers adopt mirror-image packing, where three of the four substituents at the stereocenter lie in similar positions. The fourth substituent, hydrogen, points in opposite directions.
Organizational Affiliation:
Department of Chemistry, McGill University, Montréal, Québec H3A 2K6, Canada.