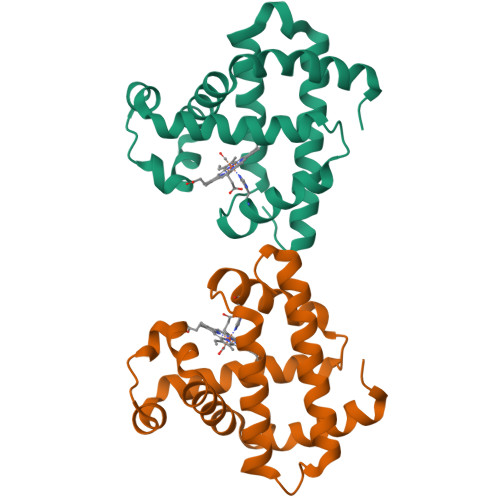
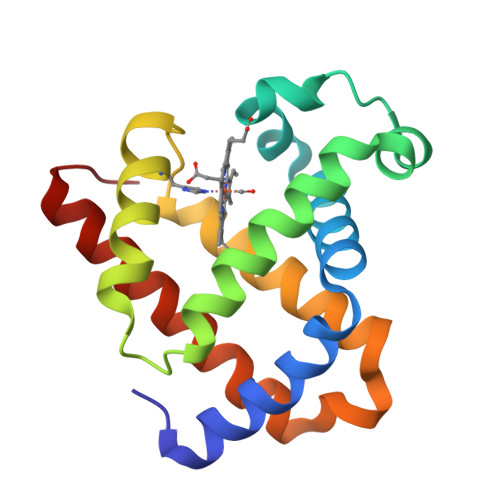
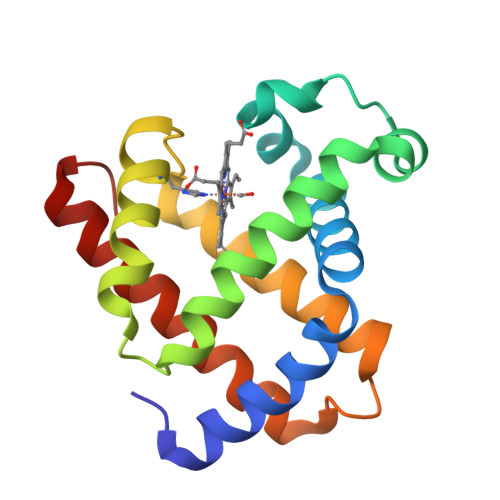
Distal pocket polarity in ligand binding to myoglobin: deoxy and carbonmonoxy forms of a threonine68(E11) mutant investigated by X-ray crystallography and infrared spectroscopy.
Cameron, A.D., Smerdon, S.J., Wilkinson, A.J., Habash, J., Helliwell, J.R., Li, T., Olson, J.S.(1993) Biochemistry 32: 13061-13070
- PubMed: 8241160
- DOI: https://doi.org/10.1021/bi00211a016
- Primary Citation of Related Structures:
1YCA, 1YCB - PubMed Abstract:
The crystal structures of the deoxy and carbonmonoxy forms of a distal pocket myoglobin mutant in which valine68(E11) is replaced by threonine have been solved to 2.1- and 2.2-A resolution, respectively. This substitution has been shown previously to cause large decreases in the rate of oxygen binding and to lower the equilibrium association constants for O2 and CO. The synchrotron Laue method was used for the rapid acquisition of X-ray diffraction data to overcome problems caused by the very rapid rate of autooxidation of the mutant protein. The refined deoxy structure shows that the noncoordinated water molecule in the distal pocket is in a position to form strong hydrogen bonds with both the N epsilon-H of the distal histidine64 and O gamma of threonine68 with no other unexpected alterations in the protein structure. In the carbonmonoxy form, the bound ligand is well-defined and inclined away from the two hydrogen-bonding groups, refining to a position in which the Fe-C-O angle is 162 degrees. This value is very close to that previously observed in recombinant wild-type and position-64 (E7) mutants of sperm whale myoglobin (160-170 degrees). The similarity of the CO conformations contrasts with the 150-fold range in equilibrium binding constants (KCO) among the distal pocket myoglobin mutants and indicates that CO affinities cannot be predicted from the coordination geometry of the bound ligand. Furthermore, a comparison of the infrared stretching frequencies of CO in wild-type, valine64 and threonine68 single mutant, and valine64-threonine68 double mutant pig carbonmonoxymyoglobins shows a lack of correlation between KCO and vCO. These effects can be understood in terms of the stability of noncovalently bound water in deoxymyoglobin and electrostatic interactions between bound ligands and the distal pocket residues.
Organizational Affiliation:
Department of Chemistry, University of York, Heslington, U.K.