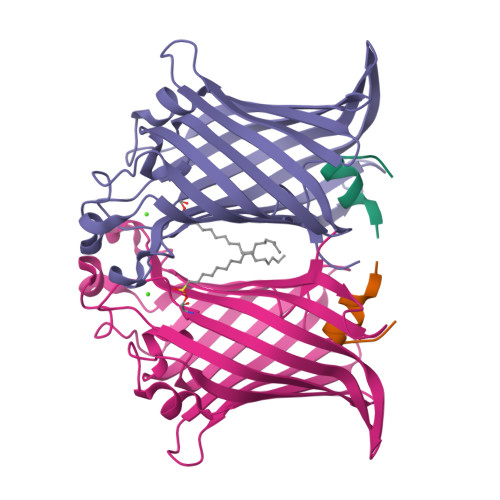
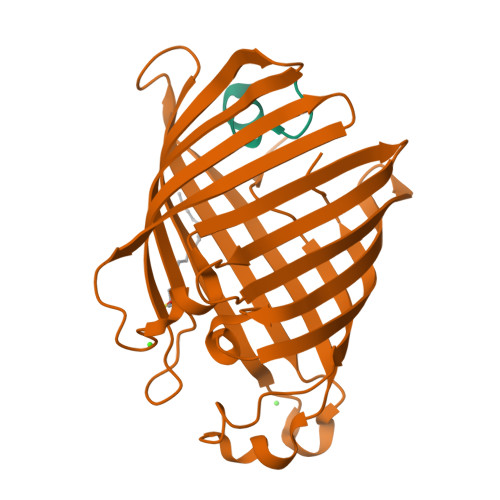
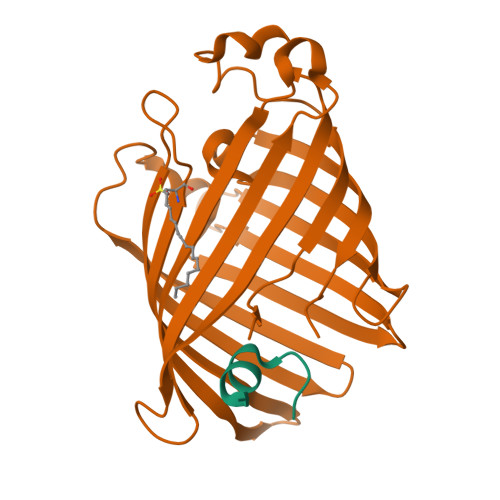
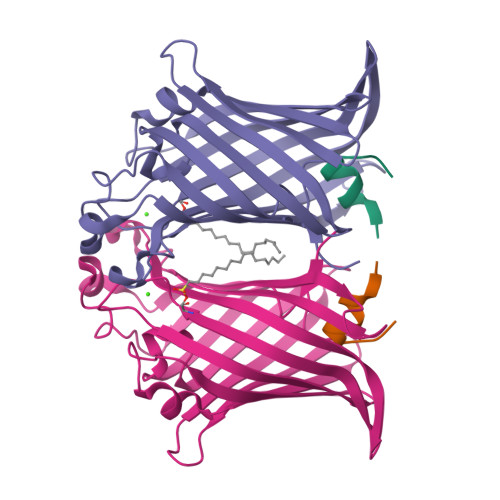
Structural evidence for dimerization-regulated activation of an integral membrane phospholipase.
Snijder, H.J., Ubarretxena-Belandia, I., Blaauw, M., Kalk, K.H., Verheij, H.M., Egmond, M.R., Dekker, N., Dijkstra, B.W.(1999) Nature 401: 717-721
- PubMed: 10537112
- DOI: https://doi.org/10.1038/44890
- Primary Citation of Related Structures:
1QD5, 1QD6 - PubMed Abstract:
Dimerization is a biological regulatory mechanism employed by both soluble and membrane proteins. However, there are few structural data on the factors that govern dimerization of membrane proteins. Outer membrane phospholipase A (OMPLA) is an integral membrane enzyme which participates in secretion of colicins in Escherichia coli. In Campilobacter and Helicobacter pylori strains, OMPLA is implied in virulence. Its activity is regulated by reversible dimerization. Here we report X-ray structures of monomeric and dimeric OMPLA from E. coli. Dimer interactions occur almost exclusively in the apolar membrane-embedded parts, with two hydrogen bonds within the hydrophobic membrane area being key interactions. Dimerization results in functional oxyanion holes and substrate-binding pockets, which are absent in monomeric OMPLA. These results provide a detailed view of activation by dimerization of a membrane protein.
Organizational Affiliation:
Laboratory of Biophysical Chemistry, BIOSON Research Institute, University of Groningen, The Netherlands.