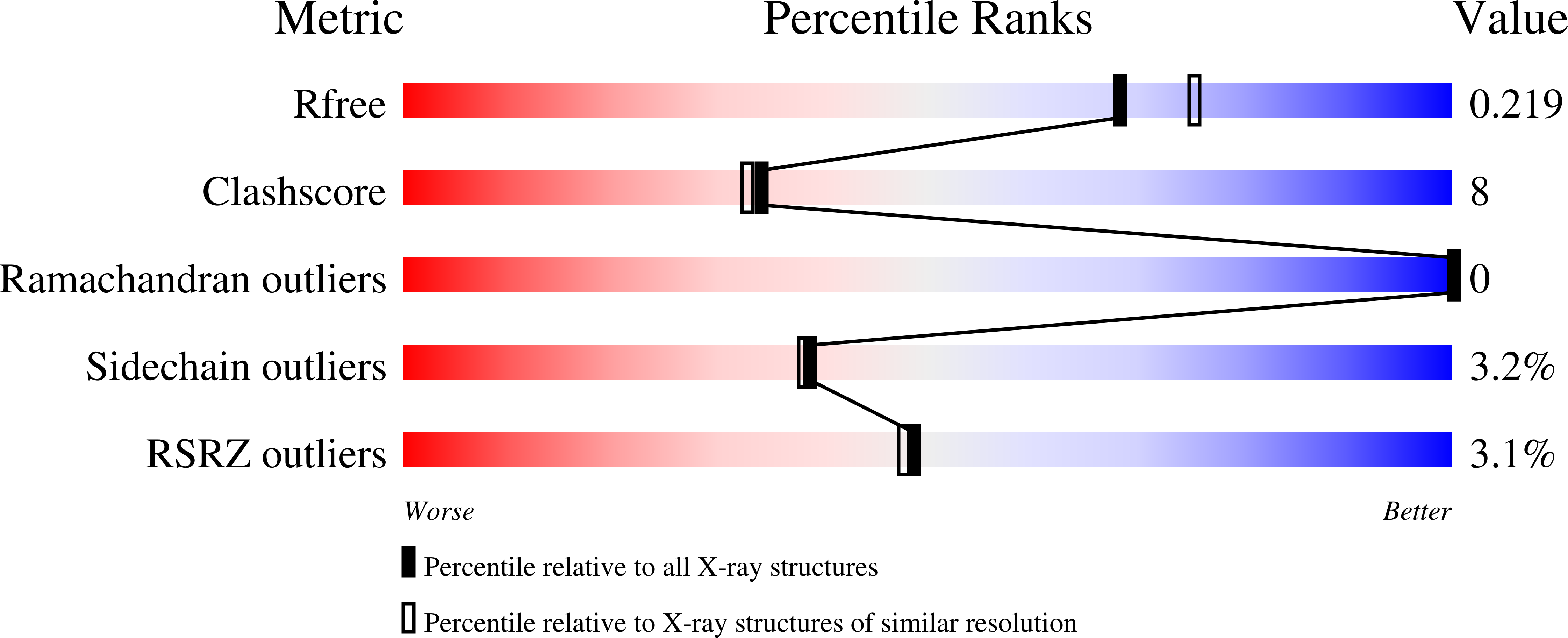
The structure of L-amino acid oxidase reveals the substrate trajectory into an enantiomerically conserved active site.
Pawelek, P.D., Cheah, J., Coulombe, R., Macheroux, P., Ghisla, S., Vrielink, A.(2000) EMBO J 19: 4204-4215
- PubMed: 10944103
- DOI: https://doi.org/10.1093/emboj/19.16.4204
- Primary Citation of Related Structures:
1F8R, 1F8S - PubMed Abstract:
The structure of L-amino acid oxidase (LAAO) from Calloselasma rhodostoma has been determined to 2.0 A resolution in the presence of two ligands: citrate and o-aminobenzoate (AB). The protomer consists of three domains: an FAD-binding domain, a substrate-binding domain and a helical domain. The interface between the substrate-binding and helical domains forms a 25 A long funnel, which provides access to the active site. Three AB molecules are visible within the funnel of the LAAO-AB complex; their orientations suggest the trajectory of the substrate to the active site. The innermost AB molecule makes hydrogen bond contacts with the active site residues, Arg90 and Gly464, and the aromatic portion of the ligand is situated in a hydrophobic pocket. These contacts are proposed to mimic those of the natural substrate. Comparison of LAAO with the structure of mammalian D-amino acid oxidase reveals significant differences in their modes of substrate entry. Furthermore, a mirror-symmetrical relationship between the two substrate-binding sites is observed which facilitates enantiomeric selectivity while preserving a common arrangement of the atoms involved in catalysis.
Organizational Affiliation:
Biochemistry Department and Montréal Joint Center for Structural Biology, McIntyre Medical Sciences Building, McGill University, 3655 Promenade Sir William Osler, Montréal, Québec, H3G 1Y6, Canada.