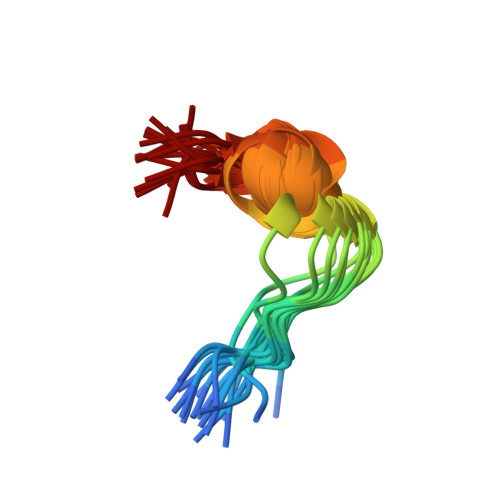
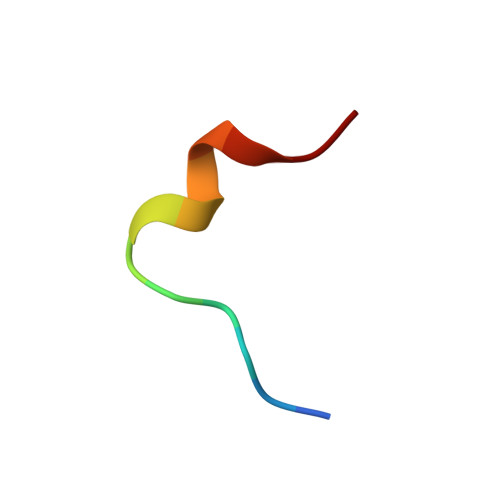
Structure of the antimicrobial peptide tritrpticin bound to micelles: a distinct membrane-bound peptide fold.
Schibli, D.J., Hwang, P.M., Vogel, H.J.(1999) Biochemistry 38: 16749-16755
- PubMed: 10606506
- DOI: https://doi.org/10.1021/bi990701c
- Primary Citation of Related Structures:
1D6X - PubMed Abstract:
Tritrpticin is a member of the cathelicidin family, a group of diverse antimicrobial peptides found in neutrophil granules. The three Trp and four Arg residues in the sequence VRRFPWWWPFLRR make this a Trp-rich cationic peptide. The structure of tritrpticin bound to membrane-mimetic sodium dodecyl sulfate micelles has been determined using conventional two-dimensional NMR methods. It forms two adjacent turns around the two Pro residues, a distinct fold for peptide-membrane interaction. The first turn involves residues 4-7, followed immediately by a second well-defined 3(10)-helical turn involving residues 8-11. The hydrophobic residues are clustered together and are clearly separated from the basic Arg residues, resulting in an amphipathic structure. Favorable interactions between the unusual amphipathic fold and the micelle surface are probably key to determining the peptide structure. NMR studies of the peptide in the micelle in the presence of the spin-label 5-doxylstearic acid determined that tritrpticin lies near the surface of the micelle, where its many aromatic side chains appear to be equally partitioned into the hydrophilic-hydrophobic interface. Additional fluorescence studies confirmed that the tryptophan residues are inserted into the micelle and are partially protected from the effects of the soluble fluorescence quencher acrylamide.
Organizational Affiliation:
Department of Biological Sciences, University of Calgary, Alberta, Canada.