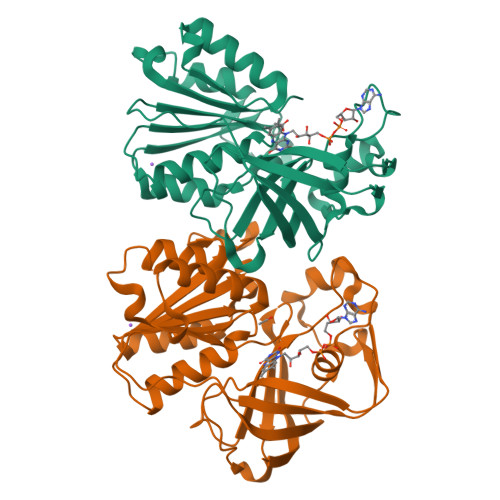
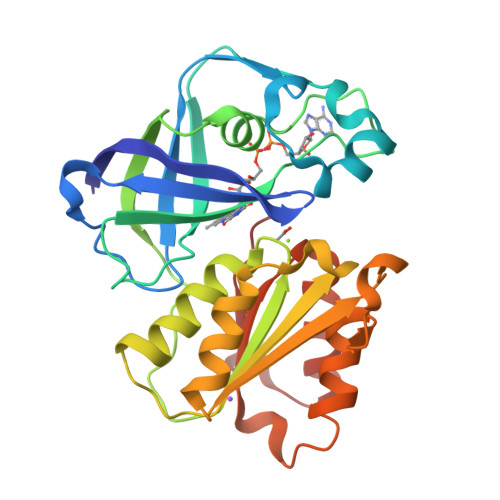
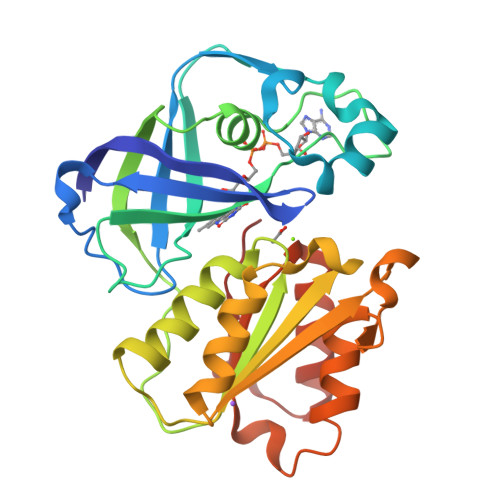
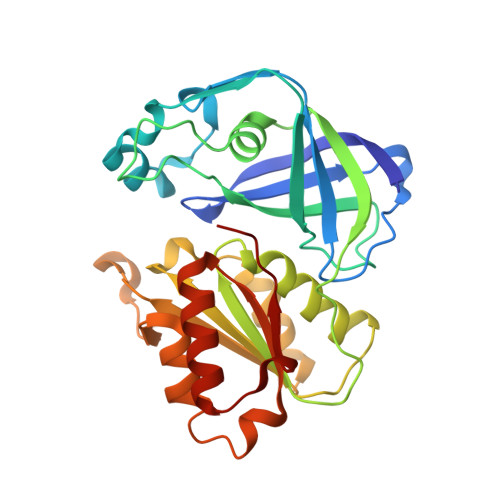
Structure of the V. cholerae Na+-pumping NADH:quinone oxidoreductase.
Steuber, J., Vohl, G., Casutt, M.S., Vorburger, T., Diederichs, K., Fritz, G.(2014) Nature 516: 62-67
- PubMed: 25471880
- DOI: https://doi.org/10.1038/nature14003
- Primary Citation of Related Structures:
4U9O, 4U9Q, 4U9S, 4U9U, 4UAJ - PubMed Abstract:
NADH oxidation in the respiratory chain is coupled to ion translocation across the membrane to build up an electrochemical gradient. The sodium-translocating NADH:quinone oxidoreductase (Na(+)-NQR), a membrane protein complex widespread among pathogenic bacteria, consists of six subunits, NqrA, B, C, D, E and F. To our knowledge, no structural information on the Na(+)-NQR complex has been available until now. Here we present the crystal structure of the Na(+)-NQR complex at 3.5 Å resolution. The arrangement of cofactors both at the cytoplasmic and the periplasmic side of the complex, together with a hitherto unknown iron centre in the midst of the membrane-embedded part, reveals an electron transfer pathway from the NADH-oxidizing cytoplasmic NqrF subunit across the membrane to the periplasmic NqrC, and back to the quinone reduction site on NqrA located in the cytoplasm. A sodium channel was localized in subunit NqrB, which represents the largest membrane subunit of the Na(+)-NQR and is structurally related to urea and ammonia transporters. On the basis of the structure we propose a mechanism of redox-driven Na(+) translocation where the change in redox state of the flavin mononucleotide cofactor in NqrB triggers the transport of Na(+) through the observed channel.
Organizational Affiliation:
Department of Microbiology, Garbenstrasse 30, University of Hohenheim, 70599 Stuttgart, Germany.