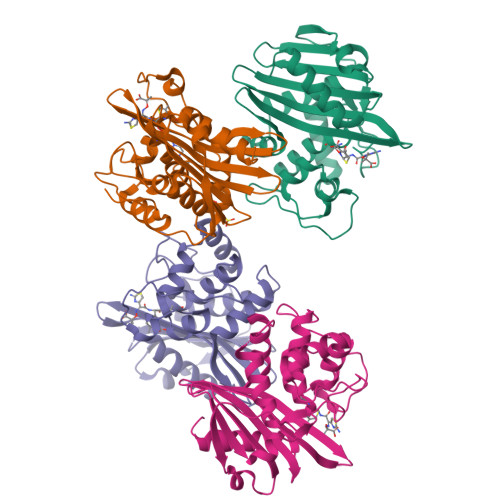
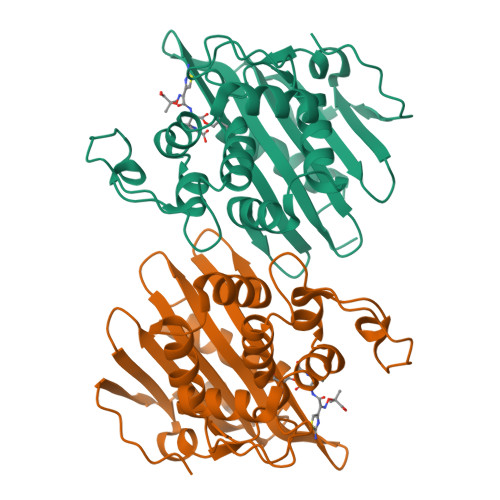
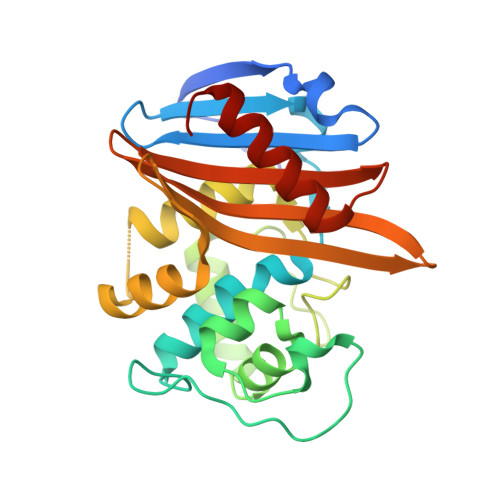
X-ray crystal structure of the acylated beta-lactam sensor domain of BlaR1 from Staphylococcus aureus and the mechanism of receptor activation for signal transduction
Birck, C., Cha, J.Y., Cross, J., Schulze-Briese, C., Meroueh, S.O., Schlegel, H.B., Mobashery, S., Samama, J.-P.(2004) J Am Chem Soc 126: 13945-13947
- PubMed: 15506754
- DOI: https://doi.org/10.1021/ja044742u
- Primary Citation of Related Structures:
1XKZ - PubMed Abstract:
Methicillin-resistant strains of Staphylococcus aureus (MRSA) are the major cause of infections worldwide. Transcription of the beta-lactamase and PBP2a resistance genes is mediated by two closely related signal-transducing integral membrane proteins, BlaR1 and MecR1, upon binding of the beta-lactam inducer to the sensor domain. Herein we report the crystal structure at 1.75 A resolution of the sensor domain of BlaR1 in complex with a cephalosporin antibiotic. Activation of the signal transducer involves acylation of serine 389 by the beta-lactam antibiotic, a process promoted by the N-carboxylated side chain of Lys392. We present evidence that, on acylation, the lysine side chain experiences a spontaneous decarboxylation that entraps the sensor in its activated state. Kinetic determinations and quantum mechanical/molecular mechanical calculations and the interaction networks in the crystal structure shed light on how this unprecedented process for activation of a receptor may be achieved and provide insights into the mechanistic features that differentiate the signal-transducing receptor from the structurally related class D beta-lactamases, enzymes of antibiotic resistance.
Organizational Affiliation:
Département de Génomique et Biologie Structurales, IGBMC CNRS/INSERM/ULP, 1 rue Laurent Fries, BP 10142, 67404 - Illkirch CU Strasbourg, France.