Distinct structural features of Pseudomonas aeruginosa ATP synthase revealed by cryo-electron microscopy.
Sobti, M., Gunn, A.P., Brown, S.H.J., Zavan, L., Fraunfelter, V.M., Wolfe, A.L., McDevitt, C.A., Steed, P.R., Stewart, A.G.(2025) Nat Commun
- PubMed: 41366214
- DOI: https://doi.org/10.1038/s41467-025-67100-0
- Primary Citation of Related Structures:
9O19, 9O1A, 9O1B, 9O1C, 9O1D, 9O1E, 9O1F, 9O1G, 9O1H, 9O1J, 9O1K - PubMed Abstract:
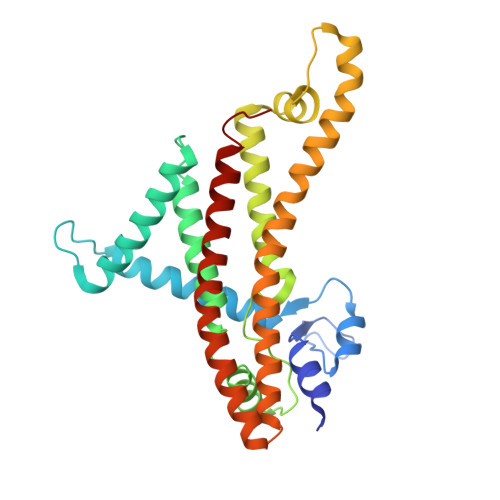
F 1 F o ATP synthase is the ubiquitous enzyme that synthesizes cellular ATP by coupling proton-motive force with rotational catalysis. Structural differences between prokaryotic and eukaryotic ATP synthases offer potential targets for antimicrobial development. Here, we present the 2.0-2.4 Å resolution cryo-electron microscopy structures of the ATP synthase from Pseudomonas aeruginosa, an opportunistic bacterial pathogen capable of causing serious infections in humans. Our structures identify two distinctive features of this species' enzyme: a distinct binding site for the inhibitory ε subunit, and a coordinated metal ion capping the cytoplasmic proton channel. Lower-resolution maps of the enzyme following incubation with MgATP showed conformational rearrangements of the ε subunit during activation. Visualization of bound water molecules in the periplasmic half-channel supports a Grotthuss proton-transfer mechanism. Focused classification of the F o motor resolves distinct ~11° sub-steps in the c-ring, corresponding to protonation and deprotonation events. Functional analyses show that modifications to either the ε subunit or the metal binding site influence ATP synthesis and hydrolysis. Mass spectrometry analyses suggests that the physiological metal within the complex is zinc. Collectively, these findings define structural features of P. aeruginosa ATP synthase that could serve as targets for antimicrobial therapeutics.
- Molecular, Structural and Computational Biology Division, The Victor Chang Cardiac Research Institute, Darlinghurst, NSW, Australia.
Organizational Affiliation: