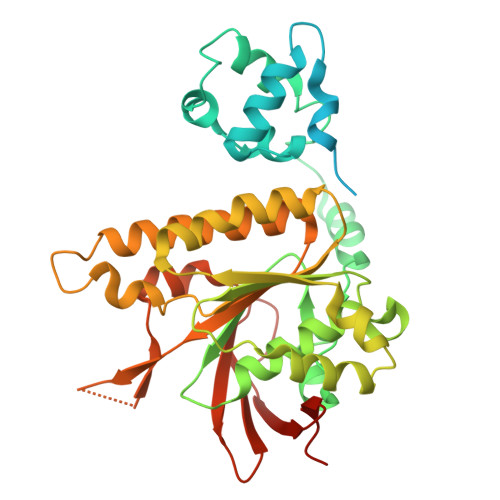
Local structural dynamics of Rad51 protomers revealed by cryo-electron microscopy of Rad51-ssDNA filaments.
Liu, J., Gore, S.K., Heyer, W.D.(2025) Nucleic Acids Res 53
- PubMed: 39898551
- DOI: https://doi.org/10.1093/nar/gkaf052
- Primary Citation of Related Structures:
9B2D, 9ED3 - PubMed Abstract:
Homologous recombination (HR) is a high-fidelity repair mechanism for double-strand breaks. Rad51 is the key enzyme that forms filaments on single-stranded DNA (ssDNA) to catalyze homology search and DNA strand exchange in recombinational DNA repair. In this study, we employed single-particle cryogenic electron microscopy (cryo-EM) to ascertain the density map of the wild-type budding yeast Rad51-ssDNA filament bound to ADP-AlF3, achieving a resolution of 2.35 Å without imposing helical symmetry. The model assigned 6 Rad51 protomers, 24 nt of DNA, and 6 bound ADP-AlF3. It shows 6-fold symmetry implying monomeric building blocks, unlike the structure of the Rad51-I345T mutant filament with three-fold symmetry implying dimeric building blocks, for which the structural comparisons provide a satisfying mechanistic explanation. This image analysis enables comprehensive comparisons of individual Rad51 protomers within the filament and reveals local conformational movements of amino acid side chains. Notably, R293 in Loop 1 adopts multiple conformations to facilitate L296 and V331 in separating and twisting the DNA triplets. We also analyzed the crystal structure of Rad51-I345T and the predicted structure of yeast Rad51-K342E using the Rad51-ssDNA structure from this study as a reference.
- Department of Microbiology & Molecular Genetics, University of California, Davis, Davis, CA 95616-8665, United States.
Organizational Affiliation: