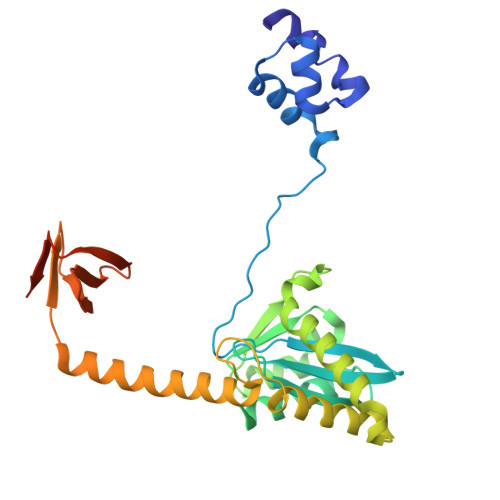
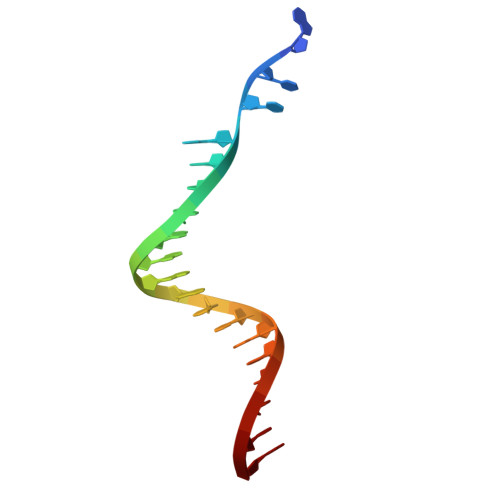
Oligomeric HIV-1 integrase structures reveal functional plasticity for intasome assembly and RNA binding.
Jing, T., Shan, Z., Dinh, T., Biswas, A., Jang, S., Greenwood, J., Li, M., Zhang, Z., Gray, G., Shin, H.J., Zhou, B., Passos, D., Strutzenberg, T.S., Aiyer, S., Andrade, L., Zhang, Y., Li, Z., Craigie, R., Engelman, A.N., Kvaratskhelia, M., Lyumkis, D.(2025) Nat Commun 16: 9430-9430
- PubMed: 41136407
- DOI: https://doi.org/10.1038/s41467-025-64479-8
- Primary Citation of Related Structures:
9BW9, 9C29 - PubMed Abstract:
Integrase (IN) performs dual essential roles during HIV-1 replication. During ingress, IN functions within an oligomeric "intasome" assembly to catalyze viral DNA integration into host chromatin. During late stages of infection, tetrameric IN binds viral RNA and orchestrates the condensation of ribonucleoprotein complexes into the capsid core. The molecular architectures of HIV-1 IN assemblies that mediate these distinct events remain unknown. Furthermore, the IN tetramer is an important antiviral target for investigational allosteric IN inhibitors. Here, we determined cryo-EM structures of wildtype HIV-1 IN tetramers and intasome hexadecamers. Our structures unveil a remarkable plasticity that leverages IN C-terminal domains and abutting linkers to assemble functionally distinct oligomeric forms. Alteration of a newly recognized conserved interface revealed that both IN functions track with tetramerization in vitro and during HIV-1 infection. Collectively, our findings reveal how IN plasticity orchestrates its diverse molecular functions and suggest a working model for IN-viral RNA binding. Moreover, our structure of the IN tetramer provides atomic blueprints for the rational development of improved allosteric inhibitors.
- The Salk Institute for Biological Studies, La Jolla, CA, USA.
Organizational Affiliation: