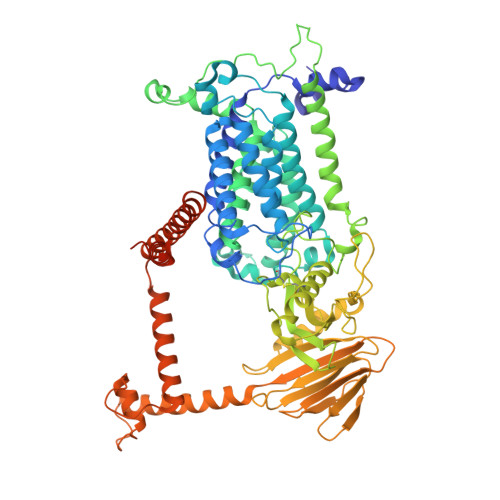
Molecular basis of vitamin-K-driven gamma-carboxylation at the membrane interface.
Cao, Q., Ammerman, A., Saimi, M., Lin, Z., Shen, G., Chen, H., Sun, J., Chai, M., Liu, S., Hsu, F.F., Krezel, A.M., Gross, M.L., Xu, J., Garcia, B.A., Liu, B., Li, W.(2025) Nature 639: 816-824
- PubMed: 39880037
- DOI: https://doi.org/10.1038/s41586-025-08648-1
- Primary Citation of Related Structures:
9BVK, 9BVL, 9BVM, 9BVO, 9BVP, 9BVQ, 9BVR - PubMed Abstract:
The γ-carboxylation of glutamate residues enables Ca 2+ -mediated membrane assembly of protein complexes that support broad physiological functions including hemostasis, calcium homeostasis, immune response, and endocrine regulation 1-4 . Modulating γ-carboxylation level provides prevalent treatments for hemorrhagic and thromboembolic diseases 5 . This unique posttranslational modification requires vitamin K hydroquinone (KH 2 ) to drive highly demanding reactions 6 catalyzed by the membrane-integrated γ-carboxylase (VKGC). To decipher underlying mechanisms, we determined cryo-electron microscopy structures of human VKGC in unbound form, with KH 2 and four hemostatic and non-hemostatic proteins possessing propeptides and glutamate-rich domains in different carboxylation states. VKGC recognizes substrate proteins via knob-and-hole interactions with propeptides, thereby bringing tethered glutamate-containing segments for processive carboxylation within a large chamber that provides steric control. Propeptide binding also triggers a global conformational change to signal VKGC activation. Through sequential deprotonation and KH 2 epoxidation, VKGC generates free hydroxide ion as an exceptionally strong base required to deprotonate the γ-carbon of glutamate for CO 2 addition. The diffusion of this superbase, protected and guided by a sealed hydrophobic tunnel, elegantly resolves the challenge of coupling KH 2 epoxidation to γ-carboxylation across the membrane interface. These structural insights and extensive functional experiments advance membrane enzymology and propel the development of novel treatments for γ-carboxylation disorders.
- Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. Louis, MO, USA.
Organizational Affiliation: