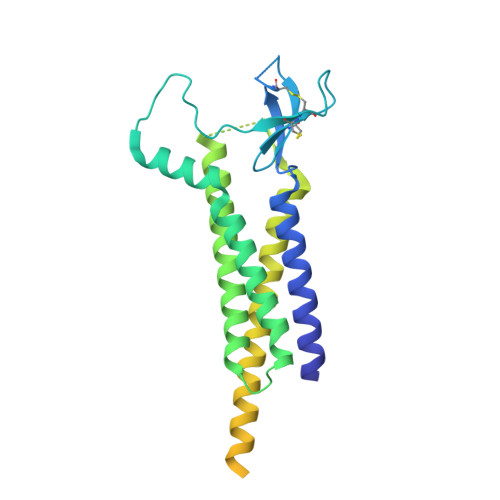
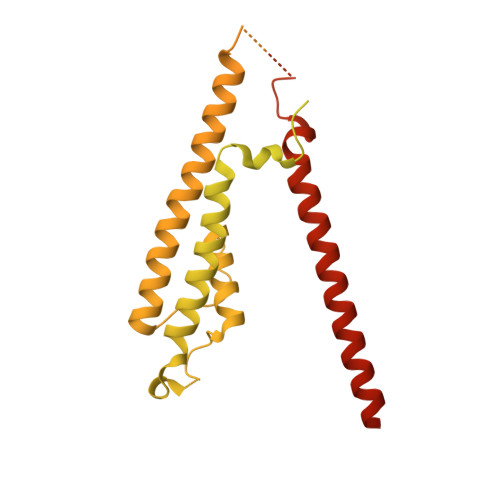
Proton-triggered rearrangement of the AMPA receptor N-terminal domains impacts receptor kinetics and synaptic localization.
Ivica, J., Kejzar, N., Ho, H., Stockwell, I., Kuchtiak, V., Scrutton, A.M., Nakagawa, T., Greger, I.H.(2024) Nat Struct Mol Biol 31: 1601-1613
- PubMed: 39138332
- DOI: https://doi.org/10.1038/s41594-024-01369-5
- Primary Citation of Related Structures:
9B5Z, 9B60, 9B61, 9B63, 9B64, 9B67, 9B68, 9B69, 9B6A - PubMed Abstract:
AMPA glutamate receptors (AMPARs) are ion channel tetramers that mediate the majority of fast excitatory synaptic transmission. They are composed of four subunits (GluA1-GluA4); the GluA2 subunit dominates AMPAR function throughout the forebrain. Its extracellular N-terminal domain (NTD) determines receptor localization at the synapse, ensuring reliable synaptic transmission and plasticity. This synaptic anchoring function requires a compact NTD tier, stabilized by a GluA2-specific NTD interface. Here we show that low pH conditions, which accompany synaptic activity, rupture this interface. All-atom molecular dynamics simulations reveal that protonation of an interfacial histidine residue (H208) centrally contributes to NTD rearrangement. Moreover, in stark contrast to their canonical compact arrangement at neutral pH, GluA2 cryo-electron microscopy structures exhibit a wide spectrum of NTD conformations under acidic conditions. We show that the consequences of this pH-dependent conformational control are twofold: rupture of the NTD tier slows recovery from desensitized states and increases receptor mobility at mouse hippocampal synapses. Therefore, a proton-triggered NTD switch will shape both AMPAR location and kinetics, thereby impacting synaptic signal transmission.
- Neurobiology Division, Medical Research Council (MRC) Laboratory of Molecular Biology, Cambridge, UK.
Organizational Affiliation: