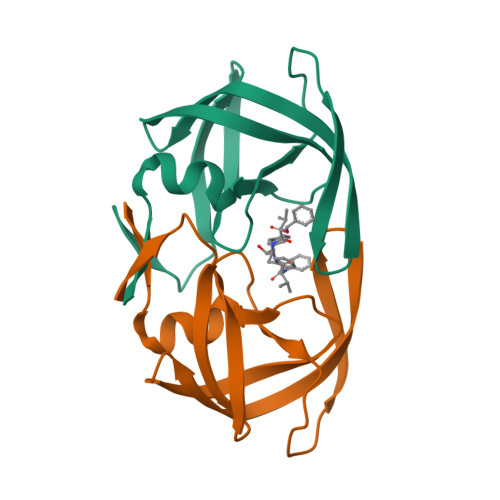
Design, activity, and 2.8 A crystal structure of a C2 symmetric inhibitor complexed to HIV-1 protease.
Erickson, J., Neidhart, D.J., VanDrie, J., Kempf, D.J., Wang, X.C., Norbeck, D.W., Plattner, J.J., Rittenhouse, J.W., Turon, M., Wideburg, N.(1990) Science 249: 527-533
- PubMed: 2200122
- DOI: https://doi.org/10.1126/science.2200122
- Primary Citation of Related Structures:
9HVP - PubMed Abstract:
A two-fold (C2) symmetric inhibitor of the protease of human immunodeficiency virus type-1 (HIV-1) has been designed on the basis of the three-dimensional symmetry of the enzyme active site. The symmetric molecule inhibited both protease activity and acute HIV-1 infection in vitro, was at least 10,000-fold more potent against HIV-1 protease than against related enzymes, and appeared to be stable to degradative enzymes. The 2.8 angstrom crystal structure of the inhibitor-enzyme complex demonstrated that the inhibitor binds to the enzyme in a highly symmetric fashion.
Organizational Affiliation:
Department of Computer-Assisted Molecular Design, Abbott Laboratories, Abbott Park, IL 60064.