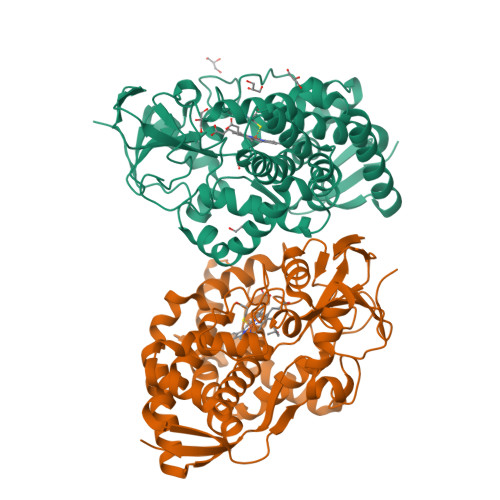
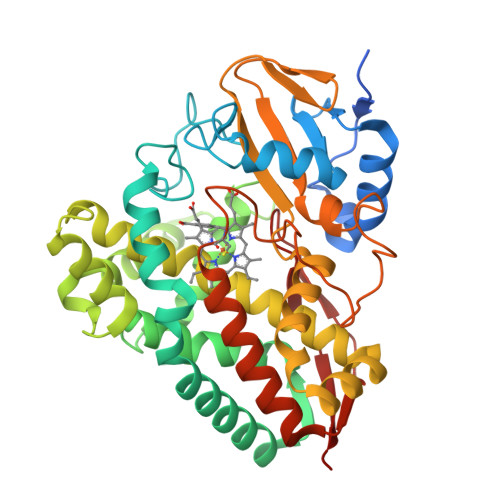
Characterization of a cytochrome P450 that catalyzes the O-demethylation of lignin-derived benzoates.
Wolf, M.E., Hinchen, D.J., McGeehan, J.E., Eltis, L.D.(2024) J Biological Chem 300: 107809-107809
- PubMed: 39307304
- DOI: https://doi.org/10.1016/j.jbc.2024.107809
- Primary Citation of Related Structures:
9G9Q, 9G9R, 9G9S - PubMed Abstract:
Cytochromes P450 (P450s) are a superfamily of heme-containing enzymes possessing a broad range of monooxygenase activities. One such activity is O-demethylation, an essential and rate-determining step in emerging strategies to valorize lignin that employ carbon-carbon bond cleavage. We recently identified PbdA, a P450 from Rhodococcus jostii RHA1, and PbdB, its cognate reductase, which catalyze the O-demethylation of para-methoxylated benzoates (p-MBAs) to initiate growth of RHA1 on these compounds. PbdA had the highest affinity (K d = 3.8 ± 0.6 μM) and apparent specificity (k cat /K M = 20 000 ± 3 000 M -1 s -1 ) for p-MBA. The enzyme also O-demethylated two related lignin-derived aromatic compounds with remarkable efficiency: veratrate and isovanillate. PbdA also catalyzed the hydroxylation and dehydrogenation of p-EB even though RHA1 did not grow on this compound. Atomic-resolution structures of PbdA in complex with p-MBA, p-EB and veratrate revealed a cluster of three residues that form hydrogen bonds with the substrates' carboxylate: Ser87, Ser237 and Arg84. Substitution of these residues resulted in lower affinity and O-demethylation activity on p-MBA as well as increased affinity for the acetyl analogue, p-methoxyacetophenone. The S87A and S237A variants of PbdA also catalyzed the O-demethylation of an aldehyde analogue of p-MBA, p-methoxy-benzaldehyde, while the R84M variant did not, despite binding this compound with high affinity. These results suggest that Ser87, Ser237 and Arg84 are not only important determinants of specificity but also help to orientate that substrate correctly in the active site. This study facilitates the design of biocatalysts for lignin valorization.
Organizational Affiliation:
Department of Microbiology and Immunology, Life Sciences Institute and Bioproducts Institute, The University of British Columbia, Vancouver, Canada.