Phalloidin and DNase I-bound F-actin pointed end structures reveal principles of filament stabilization and disassembly
Boiero Sanders, M., Oosterheert, W., Hofnagel, O., Bieling, P., Raunser, S.(2024) Nat Commun
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2024) Nat Commun
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Actin, cytoplasmic 1, N-terminally processed | 374 | Homo sapiens | Mutation(s): 1 Gene Names: ACTB | 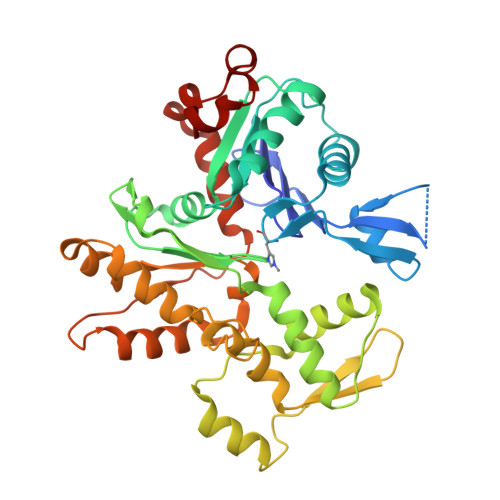 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P60709 (Homo sapiens) Explore P60709 Go to UniProtKB: P60709 | |||||
PHAROS: P60709 GTEx: ENSG00000075624 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P60709 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Phalloidin | E [auth H], F [auth I], G [auth K], H [auth J] | 7 | Amanita phalloides | Mutation(s): 0 | 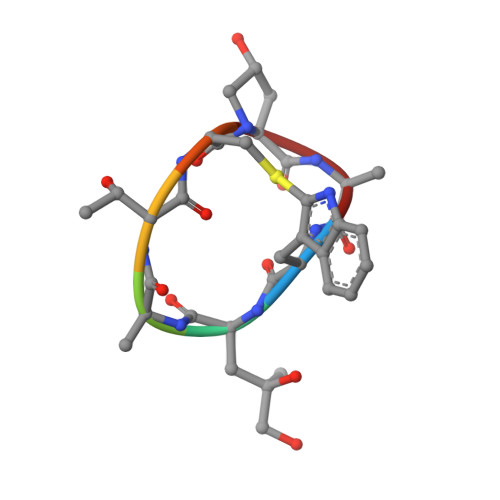 |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ADP (Subject of Investigation/LOI) Query on ADP | I [auth A], L [auth B], O [auth C], R [auth D] | ADENOSINE-5'-DIPHOSPHATE C10 H15 N5 O10 P2 XTWYTFMLZFPYCI-KQYNXXCUSA-N |  | ||
| PO4 (Subject of Investigation/LOI) Query on PO4 | K [auth A], N [auth B], Q [auth C], T [auth D] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| MG (Subject of Investigation/LOI) Query on MG | J [auth A], M [auth B], P [auth C], S [auth D] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Modified Residues 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| HIC Query on HIC | A, B, C, D | L-PEPTIDE LINKING | C7 H11 N3 O2 |  | HIS |
| HYP Query on HYP | E [auth H], F [auth I], G [auth K], H [auth J] | L-PEPTIDE LINKING | C5 H9 N O3 |  | PRO |
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_002366 Query on PRD_002366 | E [auth H], F [auth I], G [auth J], H [auth K] | Phalloidin | Peptide-like / Toxin |  | |
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | cryoSPARC | v4.2.1 |
| MODEL REFINEMENT | Coot | 0.9.8.1 |
| MODEL REFINEMENT | PHENIX | 1.21rc1_5015 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Alexander von Humboldt Foundation | Germany | -- |
| German Research Foundation (DFG) | Germany | BI 1998/2-1 |
| European Research Council (ERC) | European Union | 856118 |