Structural Studies on the Binding Mode of Bisphenols to PPAR gamma.
Useini, A., Schwerin, I.K., Kunze, G., Strater, N.(2024) Biomolecules 14
- PubMed: 38927044
- DOI: https://doi.org/10.3390/biom14060640
- Primary Citation of Related Structures:
9F7W, 9F7X - PubMed Abstract:
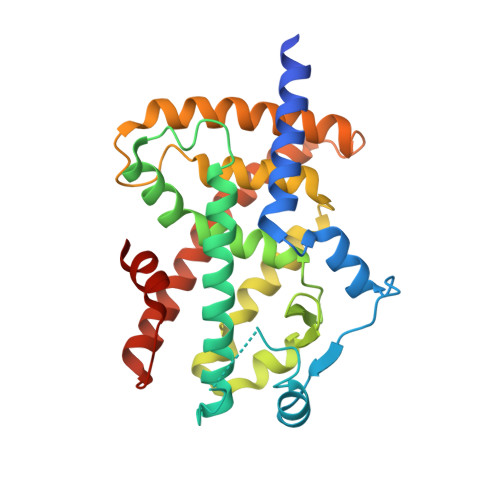
Bisphenol A (BPA) and bisphenol B (BPB) are widely used in the production of plastics, and their potential adverse health effects, particularly on endocrine disruption and metabolic health, have raised concern. Peroxisome proliferator-activated receptor gamma (PPARγ) plays a pivotal role in metabolic regulation and adipogenesis, making it a target of interest in understanding the development of obesity and associated health impacts. In this study, we employ X-ray crystallography and molecular dynamics (MD) simulations to study the interaction of PPARγ with BPA and BPB. Crystallographic structures reveal the binding of BPA and BPB to the ligand binding domain of PPARγ, next to C285, where binding of partial agonists as well as antagonists and inverse agonists of PPARγ signaling has been previously observed. However, no interaction of BPA and BPB with Y437 in the activation function 2 site is observed, showing that these ligands cannot stabilize the active conformation of helix 12 directly. Furthermore, free energy analyses of the MD simulations revealed that I341 has a large energetic contribution to the BPA and BPB binding modes characterized in this study.
Organizational Affiliation:
Institute of Bioanalytical Chemistry, Centre for Biotechnology and Biomedicine, Leipzig University, Deutscher Platz 5, 04103 Leipzig, Germany.