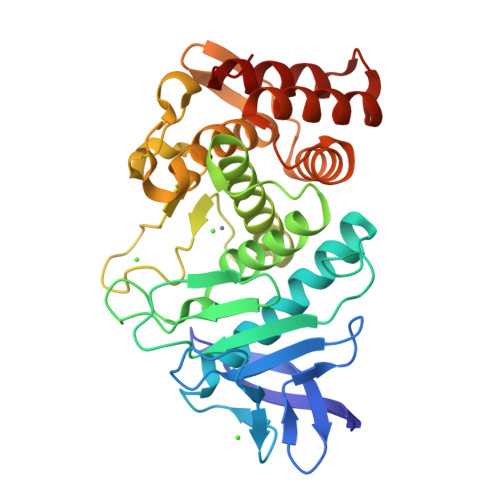
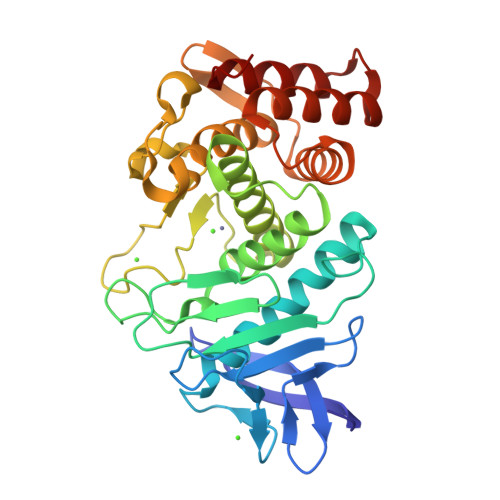
Utilizing anomalous signals for element identification in macromolecular crystallography.
El Omari, K., Forsyth, I., Duman, R., Orr, C.M., Mykhaylyk, V., Mancini, E.J., Wagner, A.(2024) Acta Crystallogr D Struct Biol 80: 713-721
- PubMed: 39291627
- DOI: https://doi.org/10.1107/S2059798324008659
- Primary Citation of Related Structures:
9F56, 9F5B, 9GCV - PubMed Abstract:
AlphaFold2 has revolutionized structural biology by offering unparalleled accuracy in predicting protein structures. Traditional methods for determining protein structures, such as X-ray crystallography and cryo-electron microscopy, are often time-consuming and resource-intensive. AlphaFold2 provides models that are valuable for molecular replacement, aiding in model building and docking into electron density or potential maps. However, despite its capabilities, models from AlphaFold2 do not consistently match the accuracy of experimentally determined structures, need to be validated experimentally and currently miss some crucial information, such as post-translational modifications, ligands and bound ions. In this paper, the advantages are explored of collecting X-ray anomalous data to identify chemical elements, such as metal ions, which are key to understanding certain structures and functions of proteins. This is achieved through methods such as calculating anomalous difference Fourier maps or refining the imaginary component of the anomalous scattering factor f''. Anomalous data can serve as a valuable complement to the information provided by AlphaFold2 models and this is particularly significant in elucidating the roles of metal ions.
Organizational Affiliation:
Diamond Light Source, Harwell Science and Innovation Campus, Didcot OX11 0DE, United Kingdom.