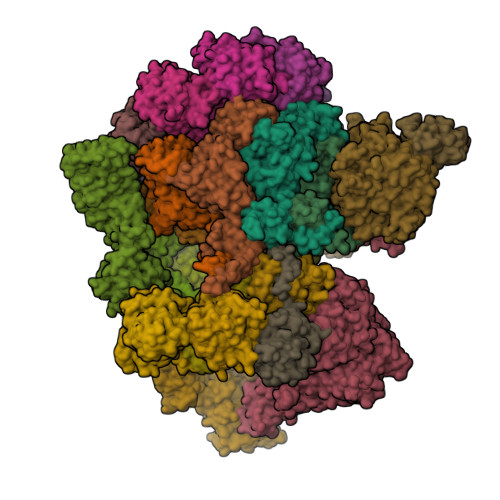
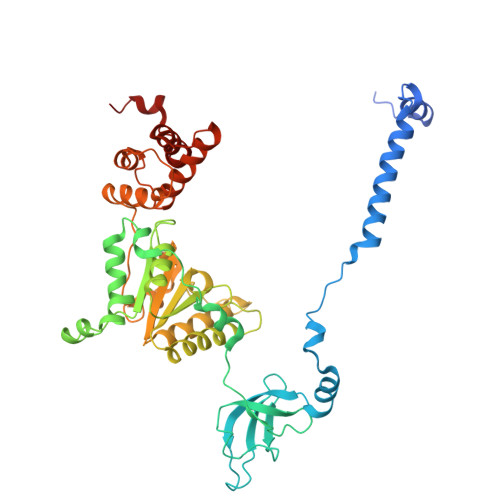
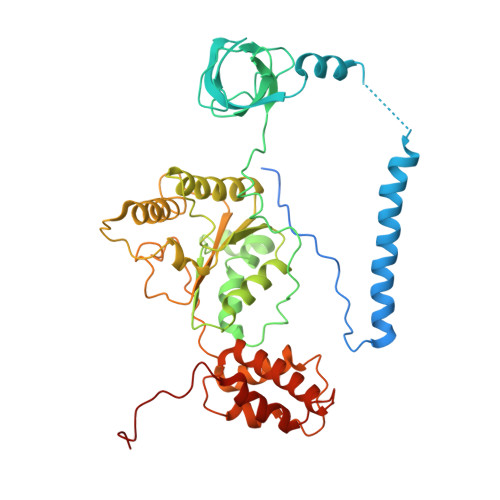
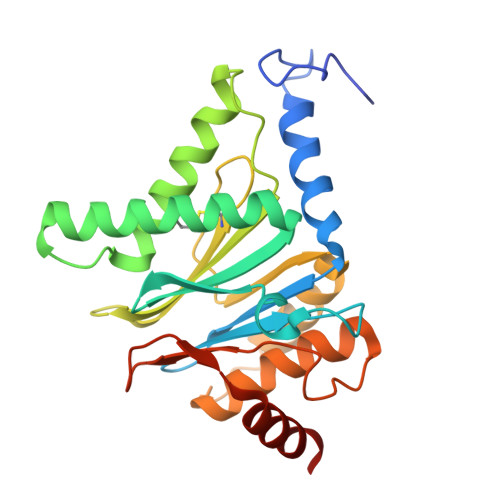
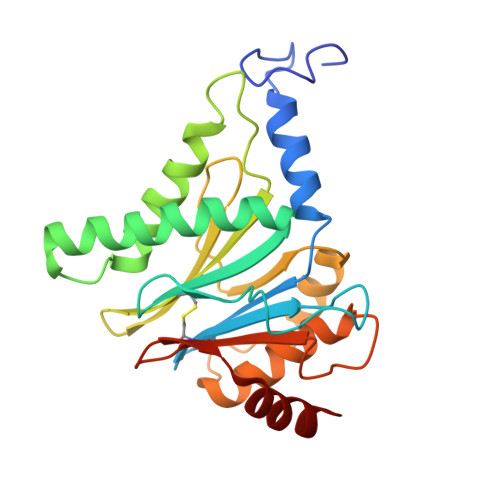
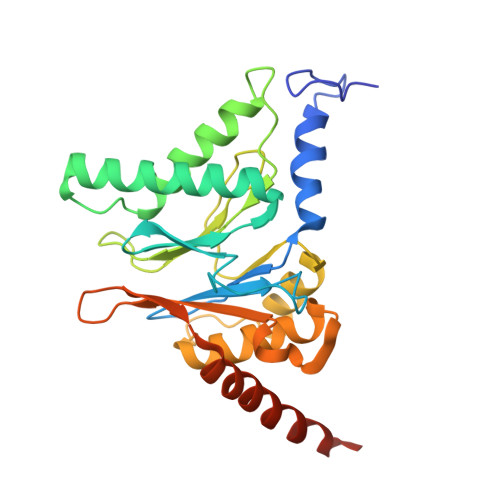
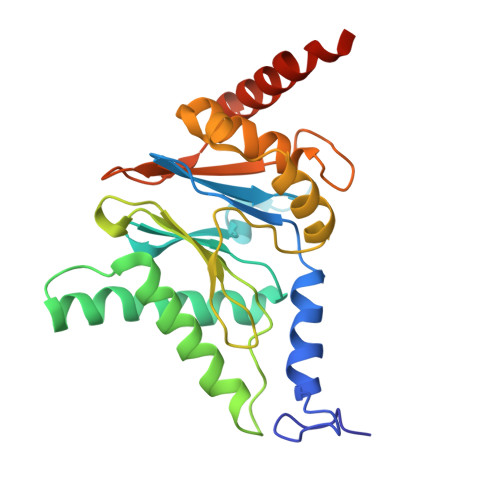
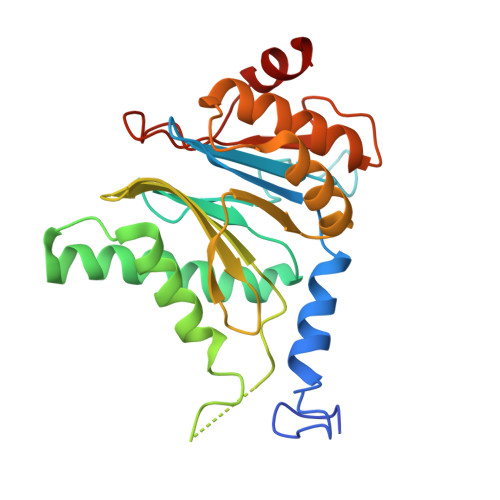
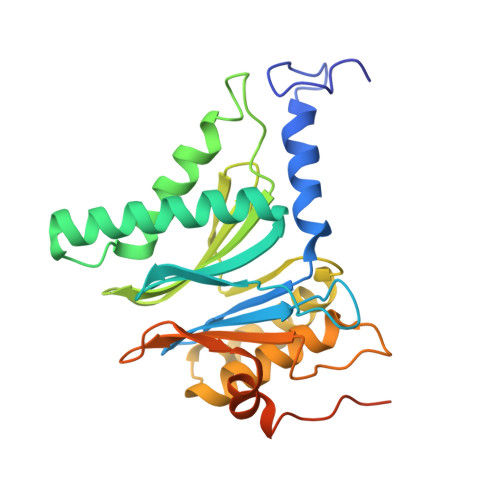
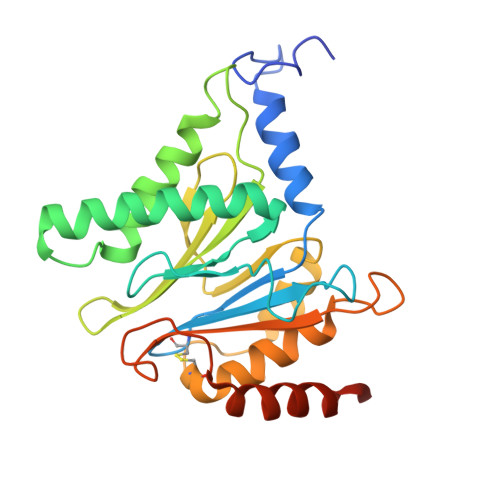
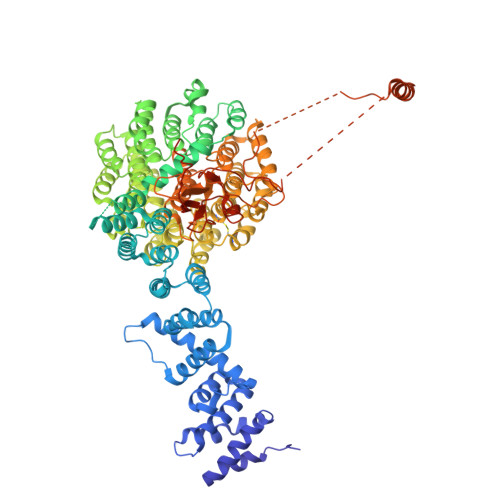
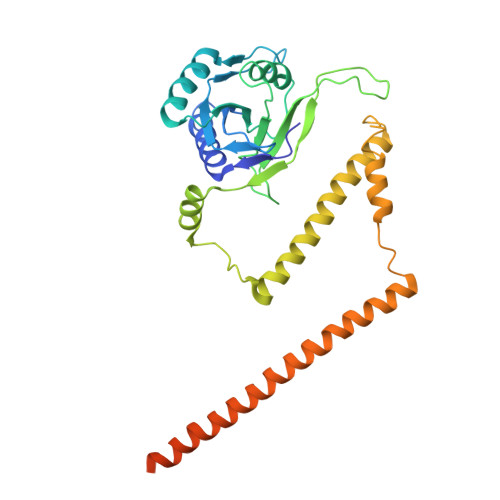
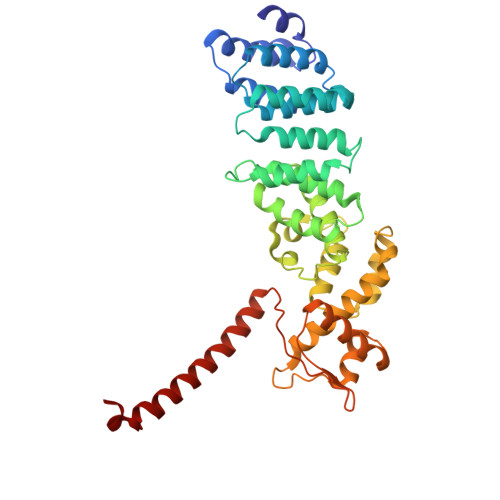
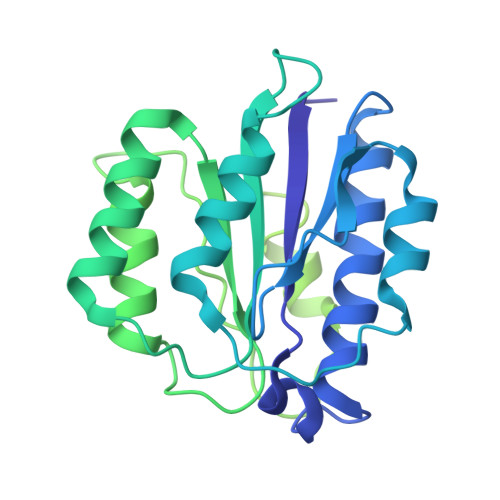
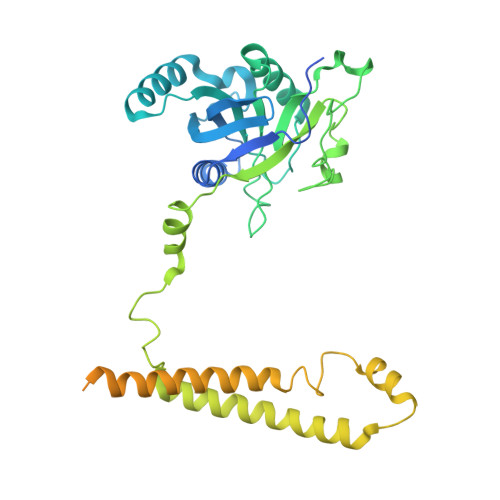
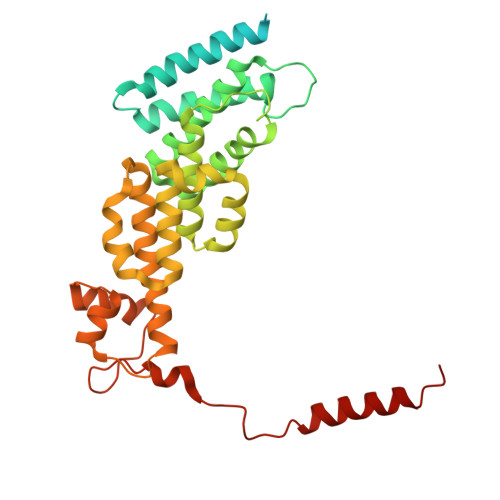
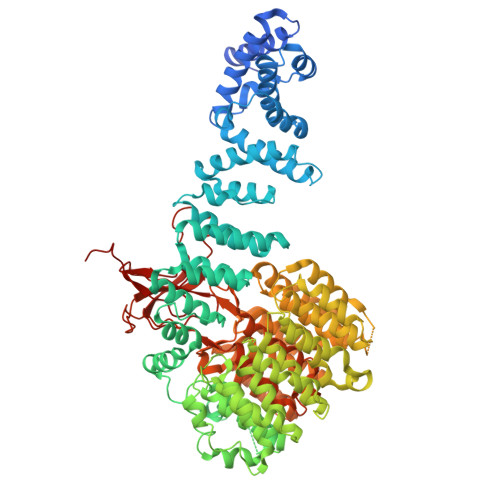
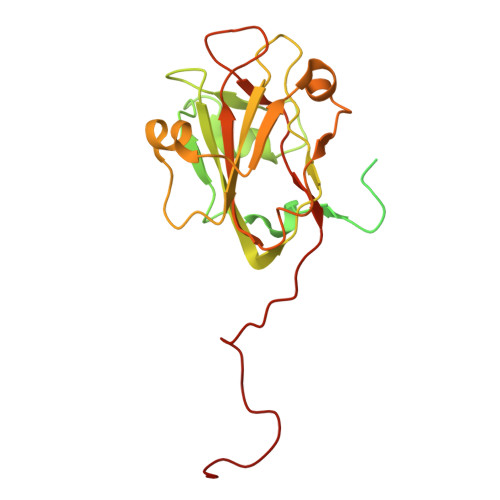
Structural landscape of AAA+ ATPase motor states in the substrate-degrading human 26S proteasome reveals conformation-specific binding of TXNL1.
Arkinson, C., Gee, C.L., Zhang, Z., Dong, K.C., Martin, A.(2024) bioRxiv
- PubMed: 39574680
- DOI: https://doi.org/10.1101/2024.11.08.622731
- Primary Citation of Related Structures:
9E8G, 9E8H, 9E8I, 9E8J, 9E8K, 9E8L, 9E8N, 9E8O, 9E8Q - PubMed Abstract:
The 26S proteasome targets many cellular proteins for degradation during general homeostasis, protein quality control, and the regulation of vital processes. A broad range of proteasome-interacting cofactors thereby modulates these functions and aids in substrate degradation. Here, we solved several high-resolution structures of the redox active cofactor TXNL1 bound to the human 26S proteasome at saturating and sub-stoichiometric concentrations by time resolved cryo-EM. We identified distinct binding modes of TXNL1 that depend on the proteasome conformational and ATPase motor states. Together with biophysical and biochemical experiments, our structural studies reveal that the resting-state proteasome prior to substrate engagement with the ATPase motor binds TXNL1 with low affinity and in variable positions on top of the Rpn11 deubiquitinase. In contrast, the actively degrading proteasome shows additional interactions leading to high-affinity TXNL1 binding, whereby TXNL1's C-terminal tail covers the catalytic groove of the Rpn11 deubiquitinase and coordinates the active-site Zn 2+ . Furthermore, these cryo-EM structures of the degrading proteasome capture the ATPase hexamer in all registers of spiral-staircase arrangements and thus visualize the complete ATP-hydrolysis cycle of the AAA+ motor, indicating temporally asymmetric hydrolysis and conformational changes in bursts during mechanical substrate unfolding and translocation. Remarkably, we catch the proteasome in the act of unfolding the beta-barrel mEos3.2 substrate while the ATPase hexamer is in a particular spiral staircase register. Our findings challenge current models for protein translocation through hexameric AAA+ motors and reveal how the proteasome uses its distinct but broad range of conformational states to coordinate cofactor binding and substrate processing.
Organizational Affiliation:
California Institute for Quantitative Biosciences, University of California at Berkeley, Berkeley, CA 94720, USA.