Structural insights into the diversity and DNA cleavage mechanism of Fanzor.
Xu, P., Saito, M., Faure, G., Maguire, S., Chau-Duy-Tam Vo, S., Wilkinson, M.E., Kuang, H., Wang, B., Rice, W.J., Macrae, R.K., Zhang, F.(2024) Cell
- PubMed: 39208796
- DOI: https://doi.org/10.1016/j.cell.2024.07.050
- Primary Citation of Related Structures:
9CER, 9CES, 9CET, 9CEU, 9CEV, 9CEW, 9CEX, 9CEY, 9CEZ, 9CF0, 9CF1, 9CF2, 9CF3 - PubMed Abstract:
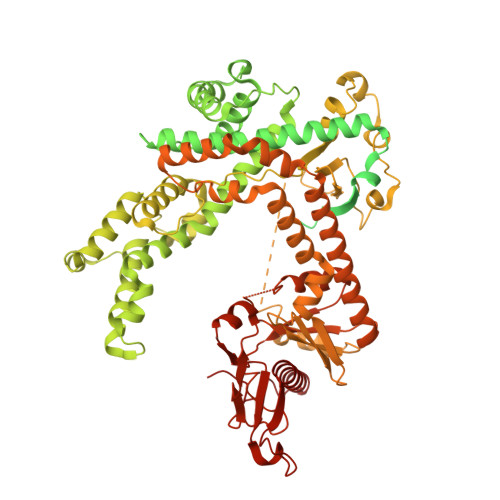
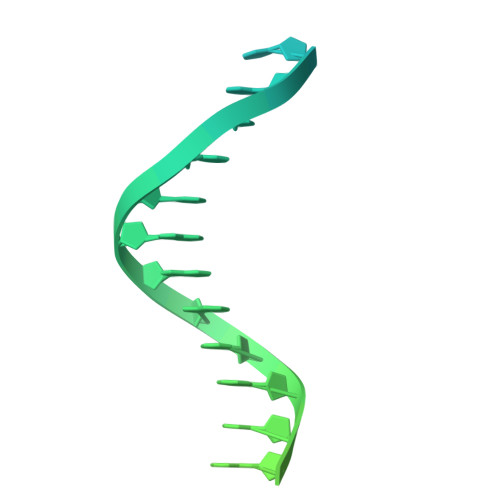
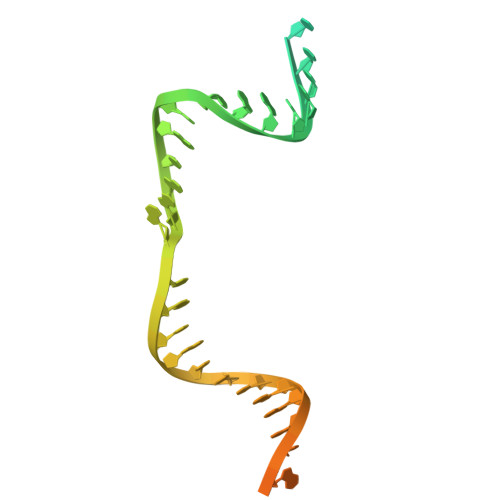
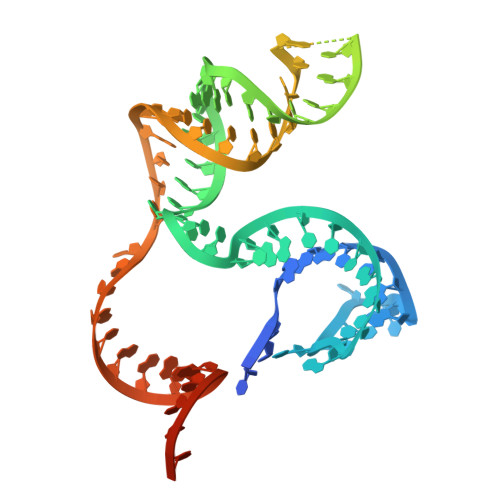
Fanzor (Fz) is an ωRNA-guided endonuclease extensively found throughout the eukaryotic domain with unique gene editing potential. Here, we describe the structures of Fzs from three different organisms. We find that Fzs share a common ωRNA interaction interface, regardless of the length of the ωRNA, which varies considerably across species. The analysis also reveals Fz's mode of DNA recognition and unwinding capabilities as well as the presence of a non-canonical catalytic site. The structures demonstrate how protein conformations of Fz shift to allow the binding of double-stranded DNA to the active site within the R-loop. Mechanistically, examination of structures in different states shows that the conformation of the lid loop on the RuvC domain is controlled by the formation of the guide/DNA heteroduplex, regulating the activation of nuclease and DNA double-stranded displacement at the single cleavage site. Our findings clarify the mechanism of Fz, establishing a foundation for engineering efforts.
Organizational Affiliation:
Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA; McGovern Institute for Brain Research at MIT, Cambridge, MA 02139, USA; Department of Brain and Cognitive Science, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Howard Hughes Medical Institute, Cambridge, MA 02139, USA.