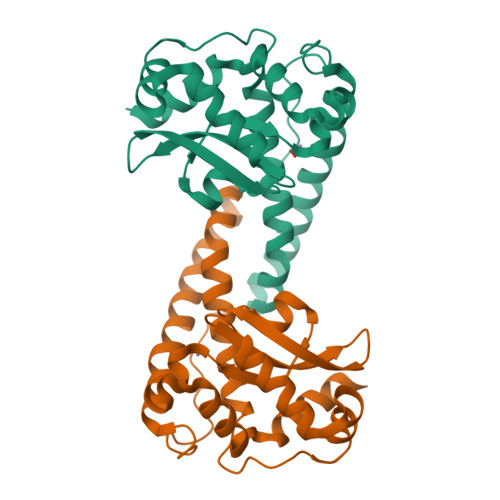
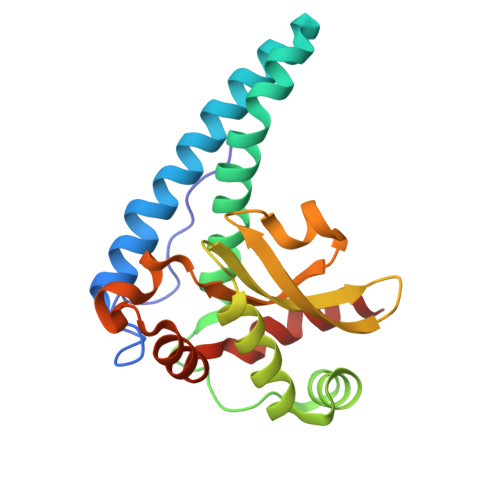
The role of Tyr34 in proton coupled electron transfer and product inhibition of manganese superoxide dismutase.
Azadmanesh, J., Slobodnik, K., Struble, L.R., Lovelace, J.J., Cone, E.A., Dasgupta, M., Lutz, W.E., Kumar, S., Natarajan, A., Coates, L., Weiss, K.L., Myles, D.A.A., Kroll, T., Borgstahl, G.E.O.(2025) Nat Commun 16: 1887-1887
- PubMed: 39987263
- DOI: https://doi.org/10.1038/s41467-025-57180-3
- Primary Citation of Related Structures:
9BVY, 9BW2, 9BWM, 9BWQ, 9BWR - PubMed Abstract:
Human manganese superoxide dismutase (MnSOD) plays a crucial role in controlling levels of reactive oxygen species (ROS) by converting superoxide (
2 ∙ - ) to molecular oxygen (O 2 ) and hydrogen peroxide (H 2 O 2 ) with proton-coupled electron transfers (PCETs). A key catalytic residue, Tyr34, determines the activity of human MnSOD and also becomes post-translationally inactivated by nitration in various diseases associated with mitochondrial dysfunction. Tyr34 has an unusual pK a due to its proximity to the Mn metal and undergoes cyclic deprotonation and protonation events to promote the electron transfers of MnSOD. Neutron diffraction, X-ray spectroscopy, and quantum chemistry calculations in oxidized, reduced and product inhibited enzymatic states shed light on the role of Tyr34 in MnSOD catalysis. The data identify the contributions of Tyr34 in MnSOD activity that support mitochondrial function and give a thorough characterization of how a single tyrosine modulates PCET catalysis. Product inhibition occurs by an associative displacement mechanism.
Organizational Affiliation:
Eppley Institute for Research in Cancer and Allied Diseases, 986805 Nebraska Medical Center, Omaha, NE, USA.