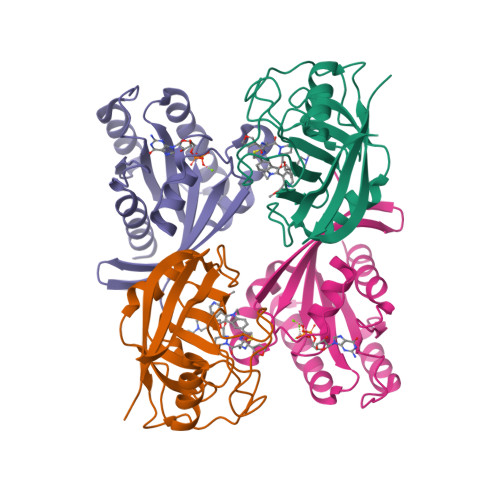
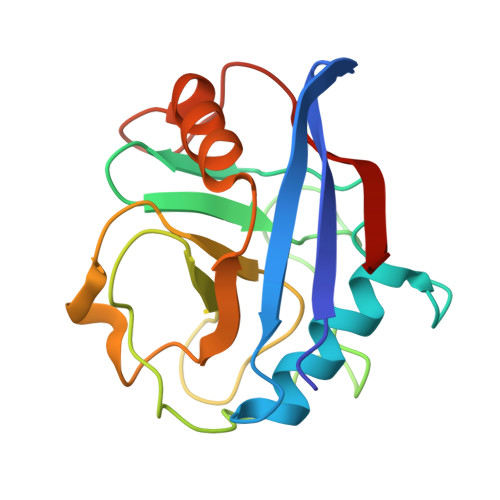
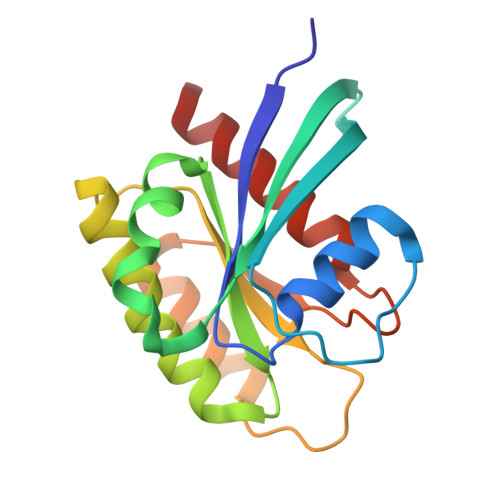
Pharmacological restoration of GTP hydrolysis by mutant RAS.
Cuevas-Navarro, A., Pourfarjam, Y., Hu, F., Rodriguez, D.J., Vides, A., Sang, B., Fan, S., Goldgur, Y., de Stanchina, E., Lito, P.(2025) Nature 637: 224-229
- PubMed: 39476862
- DOI: https://doi.org/10.1038/s41586-024-08283-2
- Primary Citation of Related Structures:
9BGH, 9BHO, 9BHP, 9BHQ, 9BI1, 9BI2 - PubMed Abstract:
Approximately 3.4 million patients worldwide are diagnosed each year with cancers that have pathogenic mutations in one of three RAS proto-oncogenes (KRAS, NRAS and HRAS) 1,2 . These mutations impair the GTPase activity of RAS, leading to activation of downstream signalling and proliferation 3-6 . Long-standing efforts to restore the hydrolase activity of RAS mutants have been unsuccessful, extinguishing any consideration towards a viable therapeutic strategy 7 . Here we show that tri-complex inhibitors-that is, molecular glues with the ability to recruit cyclophilin A (CYPA) to the active state of RAS-have a dual mechanism of action: not only do they prevent activated RAS from binding to its effectors, but they also stimulate GTP hydrolysis. Drug-bound CYPA complexes modulate residues in the switch II motif of RAS to coordinate the nucleophilic attack on the γ-phosphate of GTP in a mutation-specific manner. RAS mutants that were most sensitive to stimulation of GTPase activity were more susceptible to treatment than mutants in which the hydrolysis could not be enhanced, suggesting that pharmacological stimulation of hydrolysis potentiates the therapeutic effects of tri-complex inhibitors for specific RAS mutants. This study lays the foundation for developing a class of therapeutics that inhibit cancer growth by stimulating mutant GTPase activity.
Organizational Affiliation:
Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, New York, NY, USA.