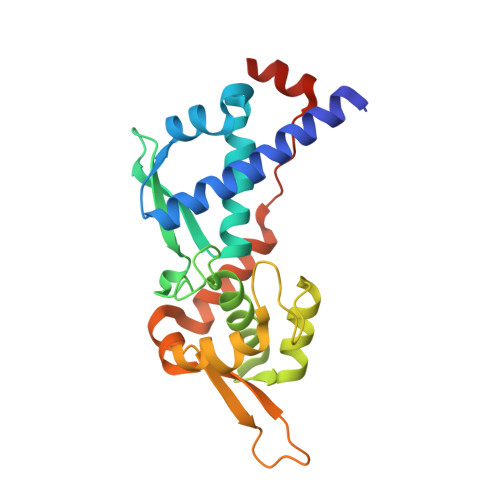
Crystal structure of MAGEA4 MHD-RAD18 R6BD reveals a flipped binding mode compared to AlphaFold2 prediction.
Forker, K., Fleming, M.C., Pearce, K.H., Vaziri, C., Bowers, A.A., Zhou, P.(2024) EMBO J 43: 2835-2839
- PubMed: 38907034
- DOI: https://doi.org/10.1038/s44318-024-00140-2
- Primary Citation of Related Structures:
9BD3
Organizational Affiliation:
Department of Biochemistry, Duke University School of Medicine, Durham, NC, 27710, USA.