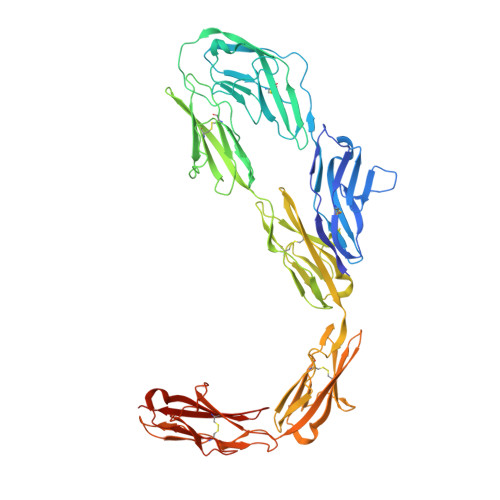
Molecular mechanism of contactin 2 homophilic interaction.
Fan, S., Liu, J., Chofflet, N., Bailey, A.O., Russell, W.K., Zhang, Z., Takahashi, H., Ren, G., Rudenko, G.(2024) Structure
- PubMed: 38968938
- DOI: https://doi.org/10.1016/j.str.2024.06.004
- Primary Citation of Related Structures:
9BA4, 9BA5 - PubMed Abstract:
Contactin 2 (CNTN2) is a cell adhesion molecule involved in axon guidance, neuronal migration, and fasciculation. The ectodomains of CNTN1-CNTN6 are composed of six Ig domains (Ig1-Ig6) and four FN domains. Here, we show that CNTN2 forms transient homophilic interactions (K D ∼200 nM). Cryo-EM structures of full-length CNTN2 and CNTN2_Ig1-Ig6 reveal a T-shaped homodimer formed by intertwined, parallel monomers. Unexpectedly, the horseshoe-shaped Ig1-Ig4 headpieces extend their Ig2-Ig3 tips outwards on either side of the homodimer, while Ig4, Ig5, Ig6, and the FN domains form a central stalk. Cross-linking mass spectrometry and cell-based binding assays confirm the 3D assembly of the CNTN2 homodimer. The interface mediating homodimer formation differs between CNTNs, as do the homophilic versus heterophilic interaction mechanisms. The CNTN family thus encodes a versatile molecular platform that supports a very diverse portfolio of protein interactions and that can be leveraged to strategically guide neural circuit development.
Organizational Affiliation:
Department of Pharmacology and Toxicology; University of Texas Medical Branch, Galveston, TX 77555, USA; Sealy Center for Structural Biology and Molecular Biophysics, University of Texas Medical Branch, Galveston, TX 77555, USA.