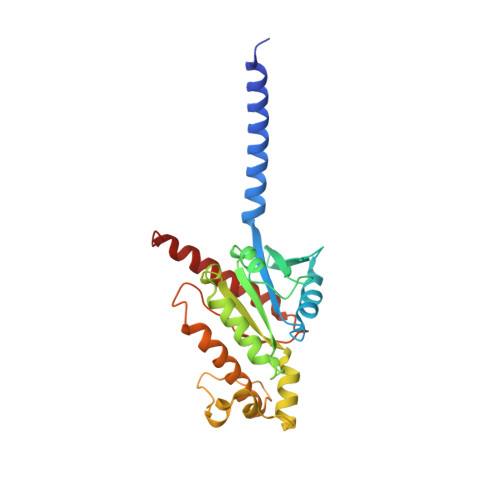
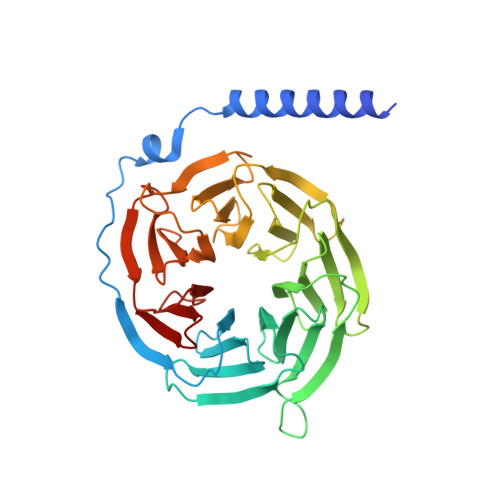
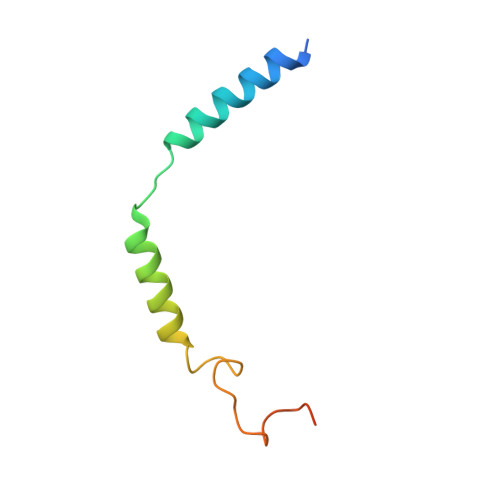
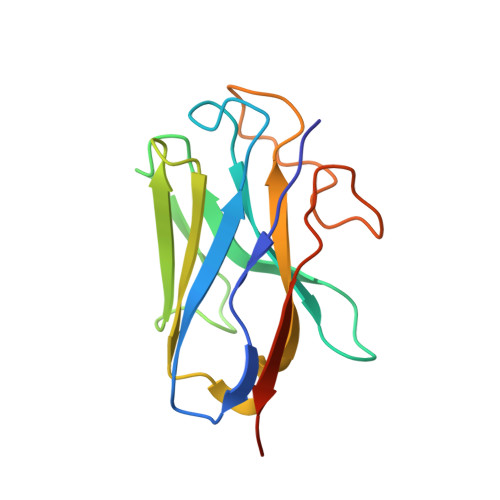
Molecular recognition and activation of the prostacyclin receptor by anti-pulmonary arterial hypertension drugs.
Wang, J.J., Jin, S., Zhang, H., Xu, Y., Hu, W., Jiang, Y., Chen, C., Wang, D.W., Xu, H.E., Wu, C.(2024) Sci Adv 10: eadk5184-eadk5184
- PubMed: 38335293
- DOI: https://doi.org/10.1126/sciadv.adk5184
- Primary Citation of Related Structures:
8X79, 8X7A - PubMed Abstract:
The prostacyclin (PGI 2 ) receptor (IP) is a G s -coupled receptor associated with blood pressure regulation, allergy, and inflammatory response. It is a main therapeutic target for pulmonary arterial hypertension (PAH) and several other diseases. Here we report cryo-electron microscopy (cryo-EM) structures of the human IP-G s complex bound with two anti-PAH drugs, treprostinil and MRE-269 (active form of selexipag), at global resolutions of 2.56 and 2.41 angstrom, respectively. These structures revealed distinct features governing IP ligand binding, receptor activation, and G protein coupling. Moreover, comparison of the activated IP structures uncovered the mechanism and key residues that determine the superior selectivity of MRE-269 over treprostinil. Combined with molecular docking and functional studies, our structures provide insight into agonist selectivity, ligand recognition, receptor activation, and G protein coupling. Our results provide a structural template for further improving IP-targeting drugs to reduce off-target activation of prostanoid receptors and adverse effects.
- State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Organizational Affiliation: