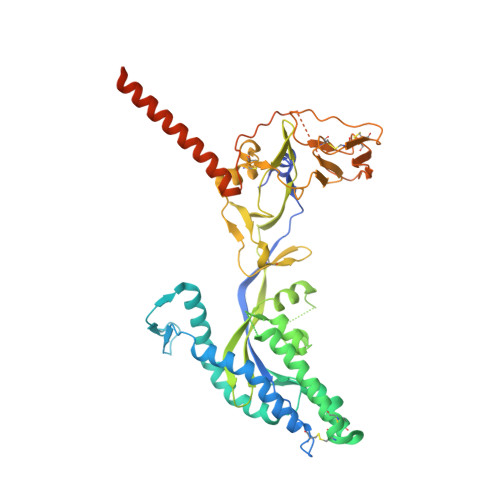
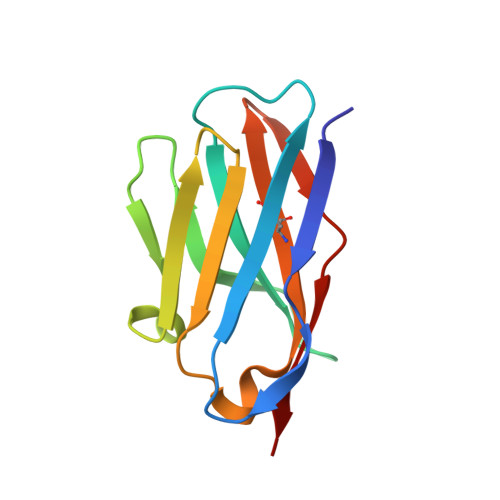
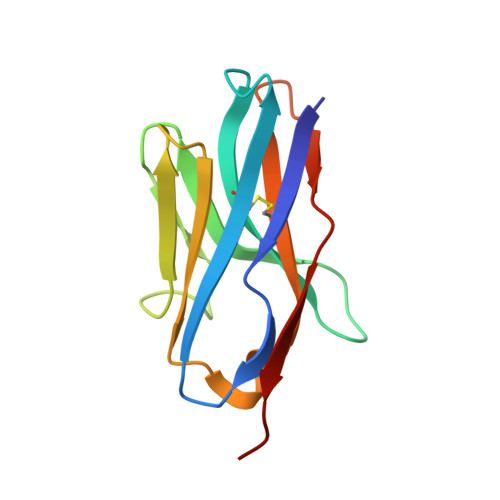
Structural basis for potent neutralization of human respirovirus type 3 by protective single-domain camelid antibodies.
Johnson, N.V., van Scherpenzeel, R.C., Bakkers, M.J.G., Ramamohan, A.R., van Overveld, D., Le, L., Langedijk, J.P.M., Kolkman, J.A., McLellan, J.S.(2024) Nat Commun 15: 5458-5458
- PubMed: 38937429
- DOI: https://doi.org/10.1038/s41467-024-49757-1
- Primary Citation of Related Structures:
8V5K, 8V62 - PubMed Abstract:
Respirovirus 3 is a leading cause of severe acute respiratory infections in vulnerable human populations. Entry into host cells is facilitated by the attachment glycoprotein and the fusion glycoprotein (F). Because of its crucial role, F represents an attractive therapeutic target. Here, we identify 13 F-directed heavy-chain-only antibody fragments that neutralize recombinant respirovirus 3. High-resolution cryo-EM structures of antibody fragments bound to the prefusion conformation of F reveal three distinct, previously uncharacterized epitopes. All three antibody fragments bind quaternary epitopes on F, suggesting mechanisms for neutralization that may include stabilization of the prefusion conformation. Studies in cotton rats demonstrate the prophylactic efficacy of these antibody fragments in reducing viral load in the lungs and nasal passages. These data highlight the potential of heavy-chain-only antibody fragments as effective interventions against respirovirus 3 infection and identify neutralizing epitopes that can be targeted for therapeutic development.
- Department of Molecular Biosciences, The University of Texas at Austin, Austin, TX, 78712, USA.
Organizational Affiliation: