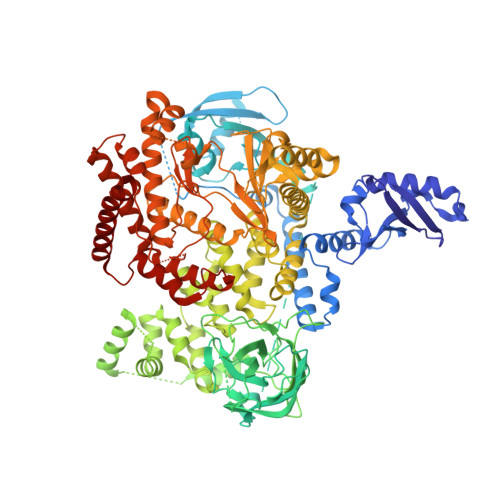
Discovery and Optimization of Pyridazinones as PI3K delta Selective Inhibitors for Administration by Inhalation.
Bruno, P., Micoli, A., Corsi, M., Pala, D., Guariento, S., Fiorelli, C., Ronchi, P., Fioni, A., Gallo, P.M., Marenghi, G., Bertolini, S., Capacchi, S., Mileo, V., Biagetti, M., Capelli, A.M.(2024) J Med Chem 67: 11103-11124
- PubMed: 38907711
- DOI: https://doi.org/10.1021/acs.jmedchem.4c00610
- Primary Citation of Related Structures:
8S3R - PubMed Abstract:
A hit-to-lead campaign pursuing the identification of novel inhalant small-molecule phosphatidylinositol 3-kinase (PI3K) inhibitors for the treatment of inflammatory respiratory diseases is disclosed. A synthetically versatile pyridazin-3(2 H )-one scaffold was designed, and three exit vectors on the core moiety were used to explore chemical diversity and optimize pharmacological and absorption, distribution, metabolism, and excretion (ADME) properties. Desired modulation of PI3Kδ selectivity and cellular potency as well as ADME properties in view of administration by inhalation was achieved. Intratracheal administration of lead compound 26 resulted in a promising pharmacokinetic profile, thus demonstrating that the optimization strategy of in vitro profiles successfully translated to an in vivo setting.
- Medicinal Chemistry and Drug Design Technologies Department, Chiesi Farmaceutici S.p.A, Nuovo Centro Ricerche, Largo Belloli 11/a, 43122 Parma, Italy.
Organizational Affiliation: