Structural insights into IL-11-mediated signalling and human IL6ST variant-associated immunodeficiency.
Gardner, S., Jin, Y., Fyfe, P.K., Voisin, T.B., Bellon, J.S., Pohler, E., Piehler, J., Moraga, I., Bubeck, D.(2024) Nat Commun 15: 2071-2071
- PubMed: 38453915
- DOI: https://doi.org/10.1038/s41467-024-46235-6
- Primary Citation of Related Structures:
8QY4, 8QY5, 8QY6 - PubMed Abstract:
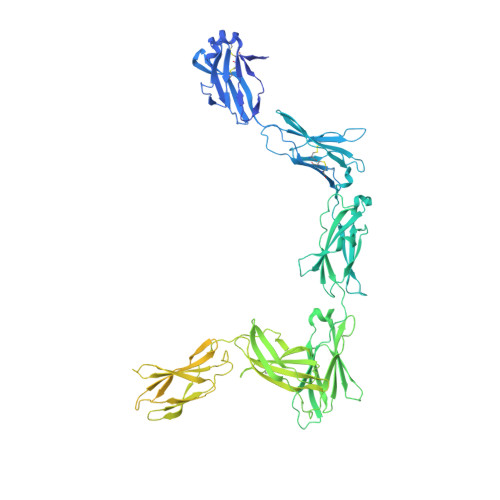
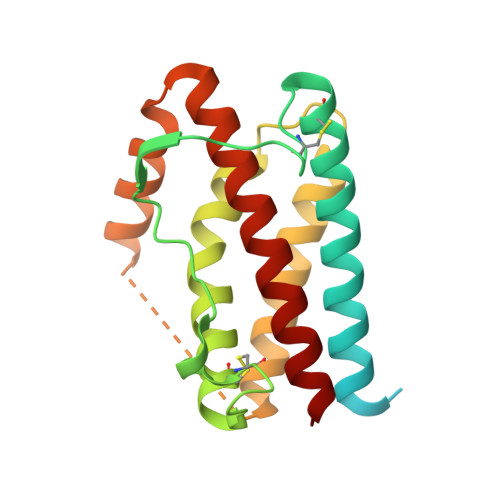
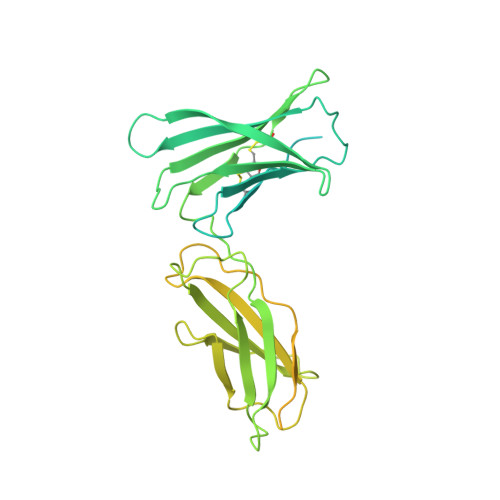
IL-11 and IL-6 activate signalling via assembly of the cell surface receptor gp130; however, it is unclear how signals are transmitted across the membrane to instruct cellular responses. Here we solve the cryoEM structure of the IL-11 receptor recognition complex to discover how differences in gp130-binding interfaces may drive signalling outcomes. We explore how mutations in the IL6ST gene encoding for gp130, which cause severe immune deficiencies in humans, impair signalling without blocking cytokine binding. We use cryoEM to solve structures of both IL-11 and IL-6 complexes with a mutant form of gp130 associated with human disease. Together with molecular dynamics simulations, we show that the disease-associated variant led to an increase in flexibility including motion within the cytokine-binding core and increased distance between extracellular domains. However, these distances are minimized as the transmembrane helix exits the membrane, suggesting a stringency in geometry for signalling and dimmer switch mode of action.
- Department of Life Sciences, Sir Ernst Chain Building, Imperial College London, London, SW7 2AZ, UK.
Organizational Affiliation: