A cyclic-nucleotide binding membrane protein provides CRISPR-mediated antiphage defence in Vibrio cholera
McMahon, S.A., McQuarrie, S., Gloster, T.M., White, M.F., Gruschow, S., Ackermann, K., Bode, B.E.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| csx23 | 96 | Vibrio cholerae | Mutation(s): 0 | 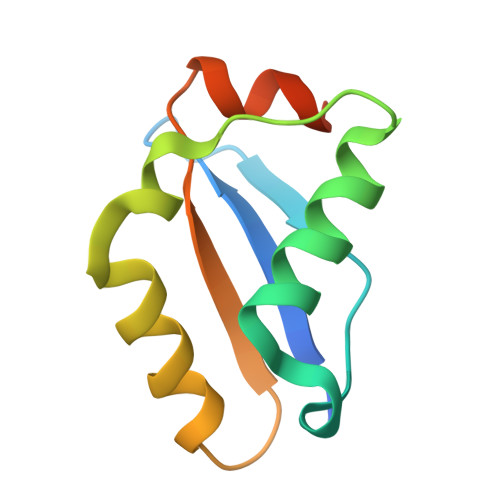 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| Cyclic tetraadenosine monophosphate (cA4) | B [auth C] | 4 | synthetic construct | 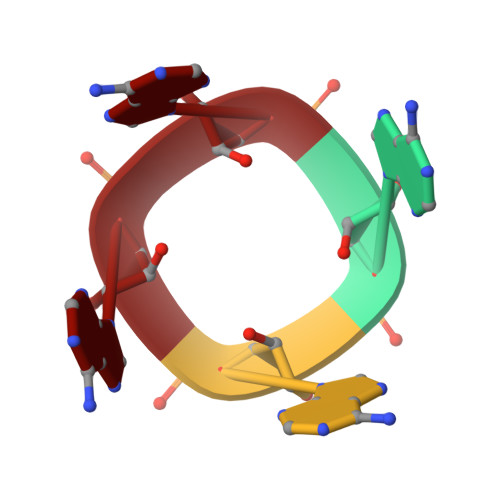 | |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ACE Query on ACE | C [auth A], D [auth A] | ACETYL GROUP C2 H4 O IKHGUXGNUITLKF-UHFFFAOYSA-N |  | ||
| NA Query on NA | E [auth C] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_002431 Query on PRD_002431 | B [auth C] | Cyclic tetraadenosine monophosphate (cA4) | Polycyclic / Antiviral |  | |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 66.425 | α = 90 |
| b = 66.425 | β = 90 |
| c = 42.479 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| autoPROC | data reduction |
| STARANISO | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Biotechnology and Biological Sciences Research Council (BBSRC) | United Kingdom | BB/T004789/1 |