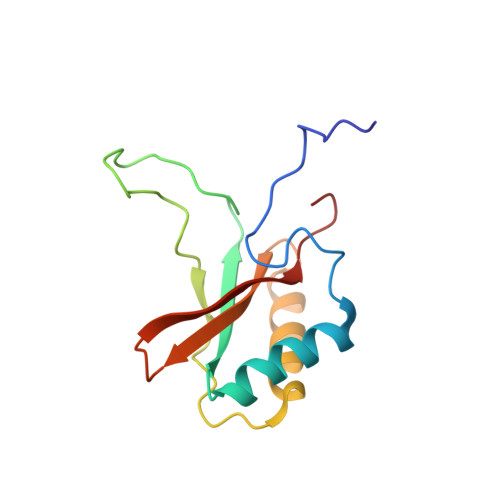
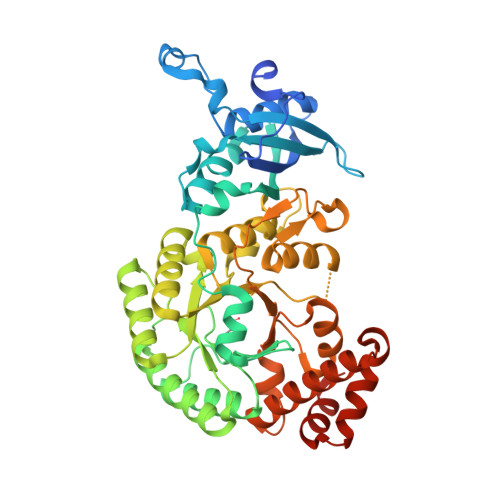
Room-temperature serial synchrotron crystallography structure of Spinacia oleracea RuBisCO.
Bjelcic, M., Aurelius, O., Nan, J., Neutze, R., Ursby, T.(2024) Acta Crystallogr F Struct Biol Commun 80: 117-124
- PubMed: 38809540
- DOI: https://doi.org/10.1107/S2053230X24004643
- Primary Citation of Related Structures:
8QJ0 - PubMed Abstract:
Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) is the enzyme responsible for the first step of carbon dioxide (CO 2 ) fixation in plants, which proceeds via the carboxylation of ribulose 1,5-biphosphate. Because of the enormous importance of this reaction in agriculture and the environment, there is considerable interest in the mechanism of fixation of CO 2 by RuBisCO. Here, a serial synchrotron crystallography structure of spinach RuBisCO is reported at 2.3 Å resolution. This structure is consistent with earlier single-crystal X-ray structures of this enzyme and the results are a good starting point for a further push towards time-resolved serial synchrotron crystallography in order to better understand the mechanism of the reaction.
- MAX IV Laboratory, Lund University, PO Box 118, 221 00 Lund, Sweden.
Organizational Affiliation: