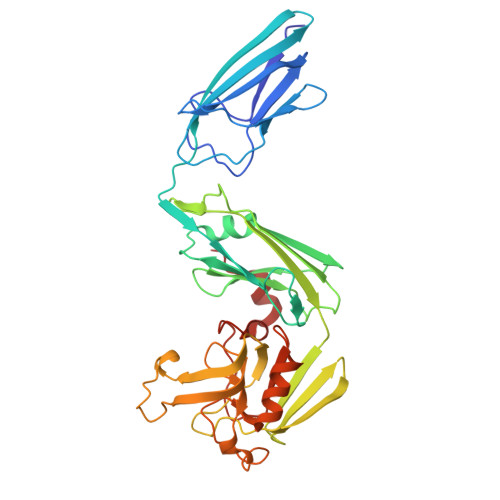
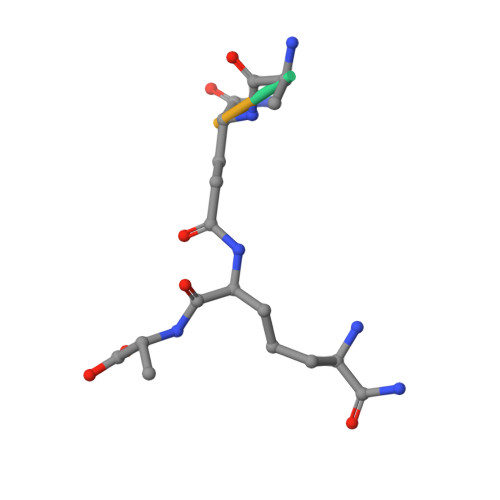
Biochemical and crystallographic studies of L,D-transpeptidase 2 from Mycobacterium tuberculosis with its natural monomer substrate.
de Munnik, M., Lang, P.A., Calvopina, K., Rabe, P., Brem, J., Schofield, C.J.(2024) Commun Biol 7: 1173-1173
- PubMed: 39294212
- DOI: https://doi.org/10.1038/s42003-024-06785-3
- Primary Citation of Related Structures:
8PXY, 8PXZ - PubMed Abstract:
The essential L,D-transpeptidase of Mycobacterium tuberculosis (Ldt Mt2 ) catalyses the formation of 3 3 cross-links in cell wall peptidoglycan and is a target for development of antituberculosis therapeutics. Efforts to inhibit Ldt Mt2 have been hampered by lack of knowledge of how it binds its substrate. To address this gap, we optimised the isolation of natural disaccharide tetrapeptide monomers from the Corynebacterium jeikeium bacterial cell wall through overproduction of the peptidoglycan sacculus. The tetrapeptides were used in binding / turnover assays and biophysical studies on Ldt Mt2. We determined a crystal structure of wild-type Ldt Mt2 reacted with its natural substrate, the tetrapeptide monomer of the peptidoglycan layer. This structure shows formation of a thioester linking the catalytic cysteine and the donor substrate, reflecting an intermediate in the transpeptidase reaction; it informs on the mode of entrance of the donor substrate into the Ldt Mt2 active site. The results will be useful in design of Ldt Mt2 inhibitors, including those based on substrate binding interactions, a strategy successfully employed for other nucleophilic cysteine enzymes.
- Chemistry Research Laboratory, Department of Chemistry and the Ineos Oxford Institute of Antimicrobial Research, University of Oxford, Oxford, UK.
Organizational Affiliation: