Structural Basis of gamma-secretase inhibition by anti-cancer clinical drugs
Guo, X., Li, H., Kai, U., Yan, C., Lei, J., Zhou, R., Shi, Y.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Presenilin-1 | A [auth B] | 467 | Homo sapiens | Mutation(s): 0 Gene Names: PSEN1, AD3, PS1, PSNL1 EC: 3.4.23 Membrane Entity: Yes | 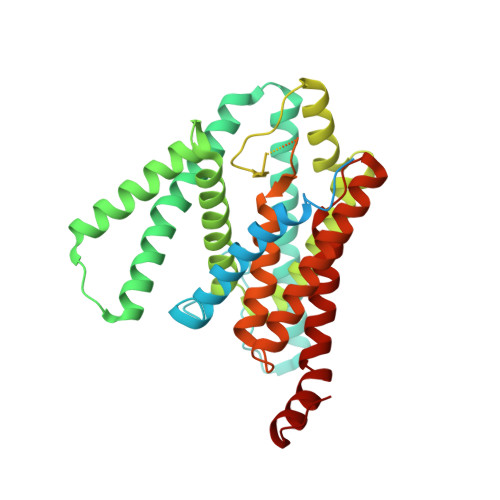 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P49768 (Homo sapiens) Explore P49768 Go to UniProtKB: P49768 | |||||
PHAROS: P49768 GTEx: ENSG00000080815 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P49768 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Nicastrin | B [auth A] | 701 | Homo sapiens | Mutation(s): 0 Gene Names: NCSTN, KIAA0253, UNQ1874/PRO4317 Membrane Entity: Yes | 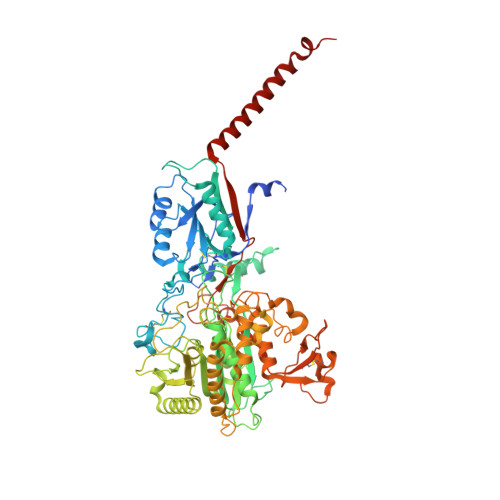 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q92542 (Homo sapiens) Explore Q92542 Go to UniProtKB: Q92542 | |||||
PHAROS: Q92542 GTEx: ENSG00000162736 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q92542 | ||||
Glycosylation | |||||
| Glycosylation Sites: 12 | Go to GlyGen: Q92542-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Gamma-secretase subunit APH-1A | 265 | Homo sapiens | Mutation(s): 0 Gene Names: APH1A, PSF, CGI-78, UNQ579/PRO1141 Membrane Entity: Yes | 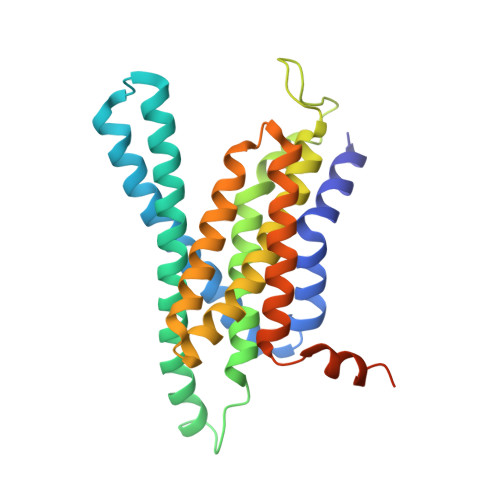 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q96BI3 (Homo sapiens) Explore Q96BI3 Go to UniProtKB: Q96BI3 | |||||
PHAROS: Q96BI3 GTEx: ENSG00000117362 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q96BI3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Gamma-secretase subunit PEN-2 | 101 | Homo sapiens | Mutation(s): 0 Gene Names: PSENEN, PEN2, MDS033 Membrane Entity: Yes | 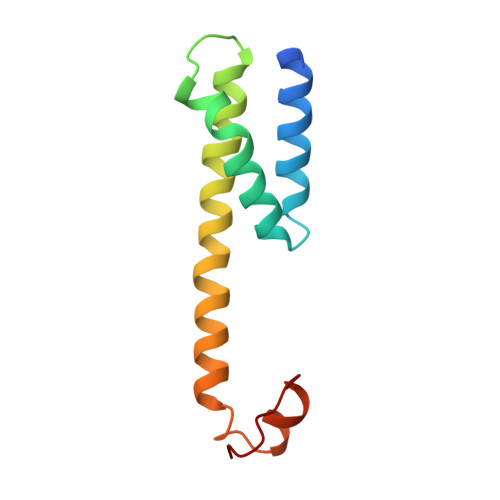 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9NZ42 (Homo sapiens) Explore Q9NZ42 Go to UniProtKB: Q9NZ42 | |||||
PHAROS: Q9NZ42 GTEx: ENSG00000205155 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9NZ42 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 5 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | E, G, H, I, J | 2 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G42666HT GlyCosmos: G42666HT GlyGen: G42666HT | |||||
Entity ID: 6 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| beta-D-mannopyranose-(1-3)-[beta-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | F | 5 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G89225LT GlyCosmos: G89225LT GlyGen: G89225LT | |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PC1 Query on PC1 | K [auth B], V [auth C] | 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE C44 H88 N O8 P NRJAVPSFFCBXDT-HUESYALOSA-N |  | ||
| OL9 (Subject of Investigation/LOI) Query on OL9 | L [auth B] | 3-[4-[2,5-bis(fluoranyl)phenyl]-4-(4-chlorophenyl)sulfonyl-cyclohexyl]propanoic acid C21 H21 Cl F2 O4 S XCGJIFAKUZNNOR-QCKZDCLWSA-N |  | ||
| CLR Query on CLR | S [auth C], T [auth C], U [auth C] | CHOLESTEROL C27 H46 O HVYWMOMLDIMFJA-DPAQBDIFSA-N |  | ||
| NAG Query on NAG | M [auth A] N [auth A] O [auth A] P [auth A] Q [auth A] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Basic Research Program of China (973 Program) | China | 2020YFA0509300 |