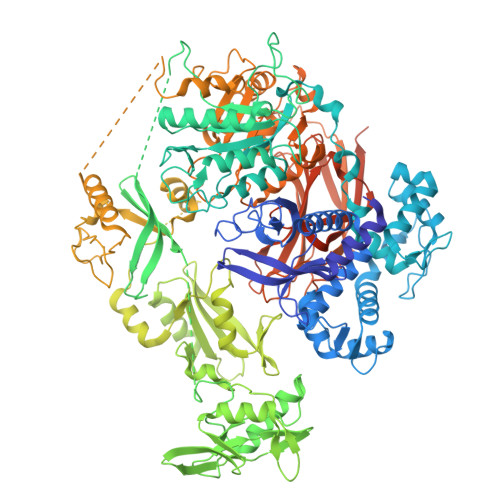
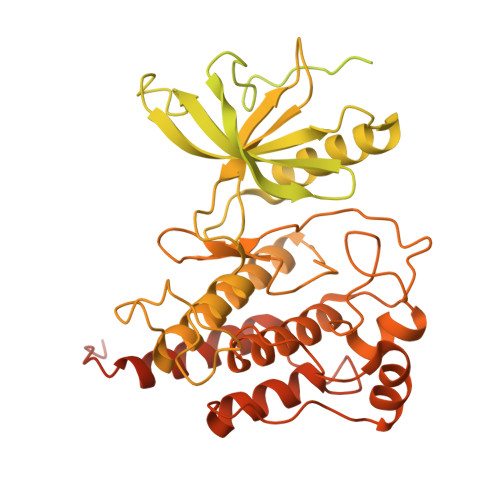
The crystal and cryo-EM structures of PLC gamma 2 reveal dynamic interdomain recognitions in autoinhibition.
Shin, Y.C., Plummer-Medeiros, A.M., Mungenast, A., Choi, H.W., TenDyke, K., Zhu, X., Shepard, J., Sanders, K., Zhuang, N., Hu, L., Qian, D., Song, K., Xu, C., Wang, J., Poda, S.B., Liao, M., Chen, Y.(2024) Sci Adv 10: eadn6037-eadn6037
- PubMed: 39612343
- DOI: https://doi.org/10.1126/sciadv.adn6037
- Primary Citation of Related Structures:
8JQG, 8JQH, 8JQI, 8T7C - PubMed Abstract:
Phospholipase C gamma 2 (PLCγ2) plays important roles in cell signaling downstream of various membrane receptors. PLCγ2 contains a multidomain inhibitory region critical for its regulation, while it has remained unclear how these domains contribute to PLCγ2 activity modulation. Here we determined three structures of human PLCγ2 in autoinhibited states, which reveal dynamic interactions at the autoinhibition interface, involving the conformational flexibility of the Src homology 3 (SH3) domain in the inhibitory region, and its previously unknown interaction with a carboxyl-terminal helical domain in the core region. We also determined a structure of PLCγ2 bound to the kinase domain of fibroblast growth factor receptor 1 (FGFR1), which demonstrates the recognition of FGFR1 by the nSH2 domain in the inhibitory region of PLCγ2. Our results provide structural insights into PLCγ2 regulation that will facilitate future mechanistic studies to understand the entire activation process.
- Department of Cell Biology, Harvard Medical School, Boston, MA 02115, USA.
Organizational Affiliation: