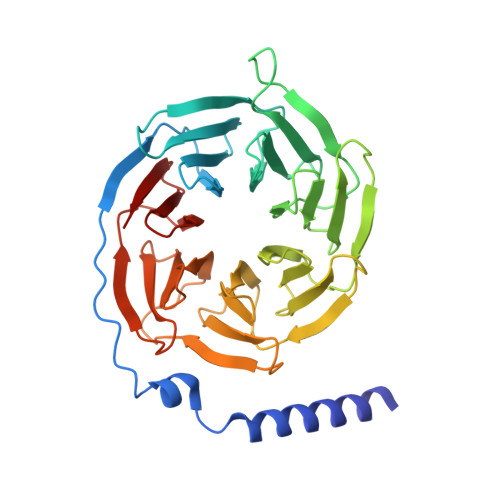
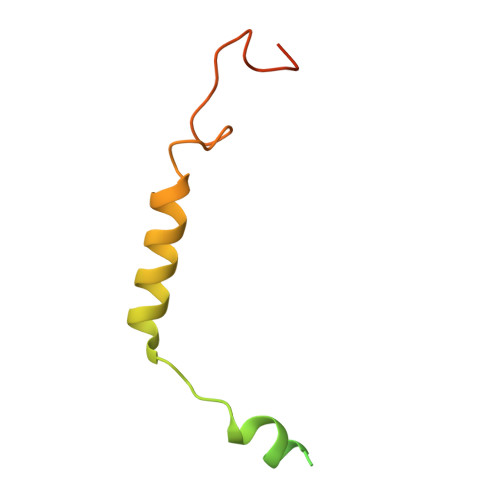
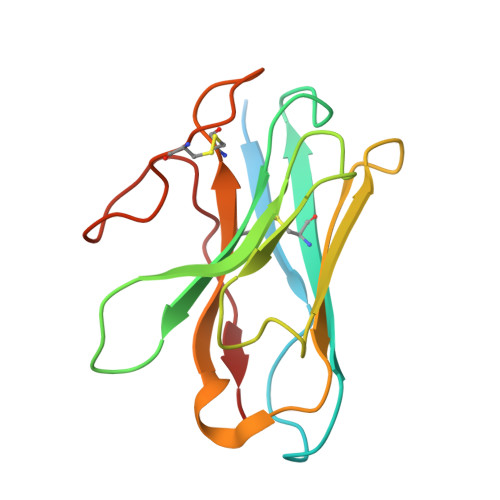
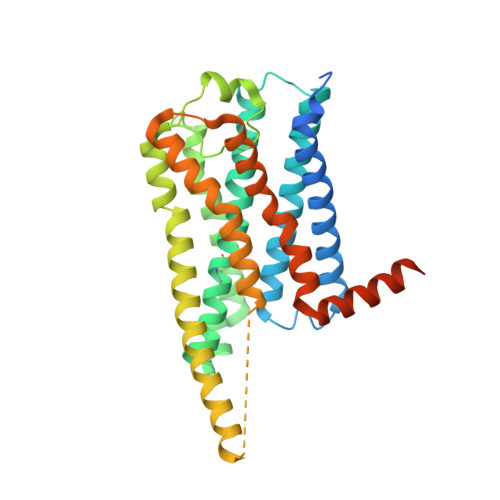
Cryo-EM structures of orphan GPR21 signaling complexes.
Lin, X., Chen, B., Wu, Y., Han, Y., Qi, A., Wang, J., Yang, Z., Wei, X., Zhao, T., Wu, L., Xie, X., Sun, J., Zheng, J., Zhao, S., Xu, F.(2023) Nat Commun 14: 216-216
- PubMed: 36639690
- DOI: https://doi.org/10.1038/s41467-023-35882-w
- Primary Citation of Related Structures:
8HIX, 8HJ0, 8HJ1, 8HJ2 - PubMed Abstract:
GPR21 is a class-A orphan G protein-coupled receptor (GPCR) and a potential therapeutic target for type 2 diabetes and other metabolic disorders. This receptor shows high basal activity in coupling to multiple G proteins in the absence of any known endogenous agonist or synthetic ligand. Here, we present the structures of ligand-free human GPR21 bound to heterotrimeric miniGs and miniG15 proteins, respectively. We identified an agonist-like motif in extracellular loop 2 (ECL2) that occupies the orthosteric pocket and promotes receptor activation. A side pocket that may be employed as a new ligand binding site was also uncovered. Remarkably, G protein binding is accommodated by a flexible cytoplasmic portion of transmembrane helix 6 (TM6) which adopts little or undetectable outward movement. These findings will enable the design of modulators for GPR21 for understanding its signal transduction and exploring opportunity for deorphanization.
- iHuman Institute, ShanghaiTech University, Pudong, Shanghai, China.
Organizational Affiliation: