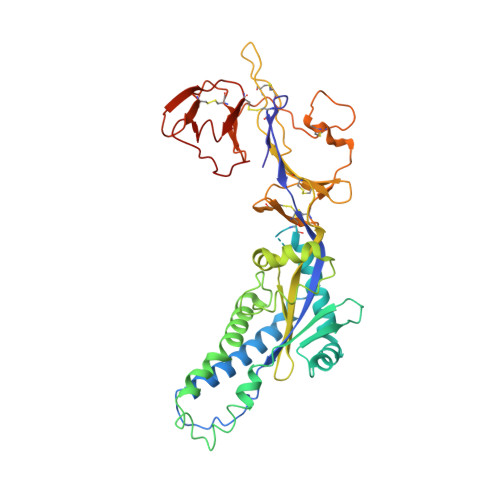
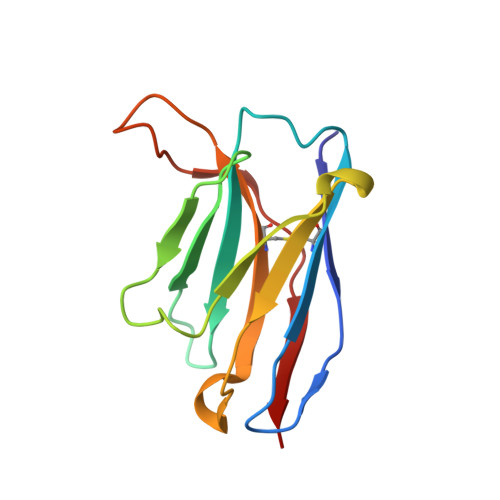
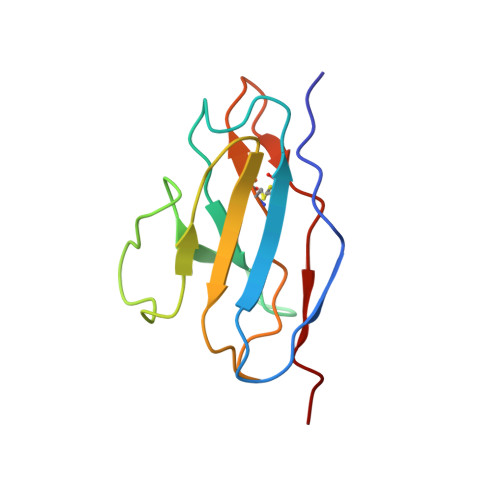
Potent cross-neutralization of respiratory syncytial virus and human metapneumovirus through a structurally conserved antibody recognition mode.
Wen, X., Suryadevara, N., Kose, N., Liu, J., Zhan, X., Handal, L.S., Williamson, L.E., Trivette, A., Carnahan, R.H., Jardetzky, T.S., Crowe Jr., J.E.(2023) Cell Host Microbe 31: 1288-1300.e6
- PubMed: 37516111
- DOI: https://doi.org/10.1016/j.chom.2023.07.002
- Primary Citation of Related Structures:
8DZW, 8E2U, 8EAY, 8EBP - PubMed Abstract:
Respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) infections pose a significant health burden. Using pre-fusion conformation fusion (F) proteins, we isolated a panel of anti-F antibodies from a human donor. One antibody (RSV-199) potently cross-neutralized 8 RSV and hMPV strains by recognizing antigenic site III, which is partially conserved in RSV and hMPV F. Next, we determined the cryoelectron microscopy (cryo-EM) structures of RSV-199 bound to RSV F trimers, hMPV F monomers, and an unexpected dimeric form of hMPV F. These structures revealed how RSV-199 engages both RSV and hMPV F proteins through conserved interactions of the antibody heavy-chain variable region and how variability within heavy-chain complementarity-determining region 3 (HCDR3) can be accommodated at the F protein interface in site-III-directed antibodies. Furthermore, RSV-199 offered enhanced protection against RSV A and B strains and hMPV in cotton rats. These findings highlight the mechanisms of broad neutralization and therapeutic potential of RSV-199.
- Department of Structural Biology, Stanford University School of Medical School, Stanford, CA 94305, USA.
Organizational Affiliation: